SUMMARY
Genome-wide association studies have identified risk loci associated with the development of inflammatory bowel disease, while epidemiological studies have emphasized that pathogenesis likely involves host interactions with environmental elements whose source and structure need to be defined. Here, we identify a class of compounds derived from dietary, microbial, and industrial sources that are characterized by the presence of a five-membered oxazole ring and induce CD1d-dependent intestinal inflammation. We observe that minimal oxazole structures modulate natural killer T cell-dependent inflammation by regulating lipid antigen presentation by CD1d on intestinal epithelial cells (IECs). CD1d-restricted production of interleukin 10 by IECs is limited through activity of the aryl hydrocarbon receptor (AhR) pathway in response to oxazole induction of tryptophan metabolites. As such, the depletion of the AhR in the intestinal epithelium abrogates oxazole-induced inflammation. In summary, we identify environmentally derived oxazoles as triggers of CD1d-dependent intestinal inflammatory responses that occur via activation of the AhR in the intestinal epithelium.
In Brief
A class of microbial and environmental compounds triggers inflammation in gut epithelial cells through the action of natural killer T cells and aryl hydrocarbon receptor signaling.
INTRODUCTION
Inflammatory bowel disease (IBD) is a complex disorder that evolves from the interactions between poorly understood environmental factors and a host’s genetic framework that together define susceptibility to and severity of disease. Pathology is influenced by specific host elements that include the autochthonous commensal microbiota, which is acquired at birth, the intestinal epithelial cell (IEC) barrier, and subjacent immune cells within the intestinal mucosa (Kaser et al., 2010). One of the great challenges of understanding IBD pathogenesis stems from efforts to elucidate the molecular details surrounding the environmental basis for these disorders. This is increasingly important, since epidemiologic studies have revealed a rapid global expansion of these diseases that includes geographic regions, which have heretofore been unaffected (Molodecky and Kaplan 2010).
A potential opportunity to investigate this question has emerged from recent studies on the role of CD1d and natural killer T (NKT) cells (NKT) in mucosal biology. CD1d is a major histocompatibility complex (MHC) class I-related molecule that presents cell-associated and microbial lipid antigens to NKT cells (Brennan et al., 2013). In the intestines, CD1d is expressed by IECs and hematopoietic cells and presents endogenous (self) or exogenous lipid antigens to NKT cells expressing an invariant T cell receptor (TCR) α chain (iNKT cells) or a semi-diverse (d) set of TCR-α chains (dNKT), which are present in human and mouse intestines (Rossjohn et al., 2012).
Experimental models using chemical induction of colitis using a classic hapten, oxazolone (Wirtz et al., 2007), implicate a role for NKT cells in the pathogenesis of IBD and have been corroborated in some patient studies (Fuss et al., 2004). Boirevant and colleagues first demonstrated that administration of oxazolone directly to the colon in ethanol of SJL/J mice, or in later studies indirectly after skin painting and sensitization, suggesting a model of haptenization, resulted in a severe acute, superficial inflammation due to the production of interleukin (IL)-4 and IL-13 that was counterbalanced by tumor growth factor β, which delimited the inflammation to the distal colon (Boirivant et al., 1998). This restriction to type 2 cytokines was eventually recognized to be genetically based as similar studies in C57BL/6 mice revealed inflammation in association with oxazolone that was due to type 1 cytokines (namely interferon γ, IFN-γ) derived from hematopoietic cells (Iijima et al., 2004). In later groundbreaking studies by Heller and colleagues, it was recognized that oxazolone-induced colitis was dependent upon CD1d and iNKT cells, as an inflammatory response to oxazolone was abrogated by the deletion of Cd1d or Jα18, encoding the invariant TCR-α chain (Heller et al., 2002) primarily derived from the activity of professional antigen-presenting cells (APCs) in the lamina propria using bone marrow chimeras (Olszak et al., 2014). In contrast, CD1d-expressing IECs were shown to secrete anti-inflammatory IL-10 in response to iNKT cells in a CD1d-dependent manner, which serves to restrain oxazolone-induced inflammation derived from the hematopoietic system (Colgan et al., 1999; Olszak et al., 2014). Thus, when CD1d-restricted pathways in the IEC are specifically eliminated by conditional genetic deletion of Cd1d, microsomal triglyceride transfer protein (Mttp), an endoplasmic reticulum resident protein that serves to regulate CD1d lipidation and its ability to function, or Il10, oxazolone-induced colitis is unrestrained and severe (Dougan et al., 2005, 2007; Sagiv et al., 2007; Olszak et al., 2014; Brozovic et al., 2004). Human studies have further identified a potential role for dNKT cells in IBD by showing elevated IL-13 production by lamina propria mononuclear cells in response to sulfatide, a CD1d-restricted self-antigen that can activate iNKT and dNKT cells (Fuss et al., 2004, 2014). Further, TCR transgenic mice expressing a non-invariant CD1d-restricted TCR develop colitis (Wang et al., 2018), though their exact role during the pathogenesis of IBD remains unknown (Grose et al., 2007; van der Vliet et al., 2001).
Importantly, host inflammatory responses to oxazolone occur even in animals raised under germ-free (GF) conditions, indicating that NKT-cell-mediated response to self (rather than microbial)-lipid antigens presented by CD1d on professional and/or non-professional APCs is sufficient for pathogenesis (Olszak et al., 2012). Instead, commensal bacteria themselves can modulate the magnitude of NKT cell responses either by directly altering the CD1d-restricted lipid antigen reservoir (An et al., 2014, Wieland Brown et al., 2013) or indirectly by regulating the quantity of iNKT cells in the colon (Olszak et al., 2012). Conversely, CD1d itself plays a critical role in regulating the composition and extent of colonization by both commensal and pathogenic microbial species potentially through disruption of the antimicrobial activity of Paneth cells (Nieuwenhuis et al., 2002, 2009).
The similarities between these biological observations in mouse models and current hypotheses for IBD pathogenesis lead us to hypothesize that oxazolone may be an example of a much larger collection of environmental chemical moieties capable of triggering CD1d-restricted iNKT cell responses. Consistent with this, we have identified environmental mimetics of oxazolone that are derived from dietary, industrial, or microbial sources that are characterized by the presence of a five-membered oxazole ring and capable of driving CD1d-dependent inflammation through activation of the aryl hydrocarbon receptor (AhR) pathway within IECs of the colon. As efforts to define and evaluate natural and synthetic chemicals involved in homeostasis or disease have generally been elusive, these results have broad implications for understanding the environmental basis of mucosal diseases, such as IBD.
RESULTS
Defining a Structural Moiety that Modulates Epithelial-Derived CD1d-Dependent Inflammatory Responses
4-ethoxymethylene-2-phenyl-2-oxazol-5-one (referred to as oxazolone; Figure 1A) has been widely utilized in models of contact hypersensitivity and, when applied to the mucosa, has been proposed to cause colitis through its properties as a hapten (Gorbachev and Fairchild 2001). However, to date, there is limited evidence for hapten-specific antibody production or responses following topical oxazolone sensitization or hapten-modified autologous proteins or luminal antigens identified at mucosal sites (Singleton et al., 2016; Wirtz et al., 2007). Interestingly, upon analysis of previously published microarray studies derived from epithelial-enriched colon fractions following intra-rectal oxazolone challenge (Olszak et al., 2014), we observed decreased expression of Mttp/MTP and increased expression for elements associated with AhR signaling, including P450 enzymes (cyp1a1) and indoleamine 2,3-dioxygenase (Ido1). We therefore asked whether oxazolone could affect the expression of these transcripts in the intestinal epithelium in a cell-intrinsic manner by directly stimulating an immortalized IECs derived from mouse small intestine, MODE-K, with oxazolone. We observed downregulation of Mttp and induction of metabolic genes cyp1a1 and Ido1 (Figures 1B and S1A).
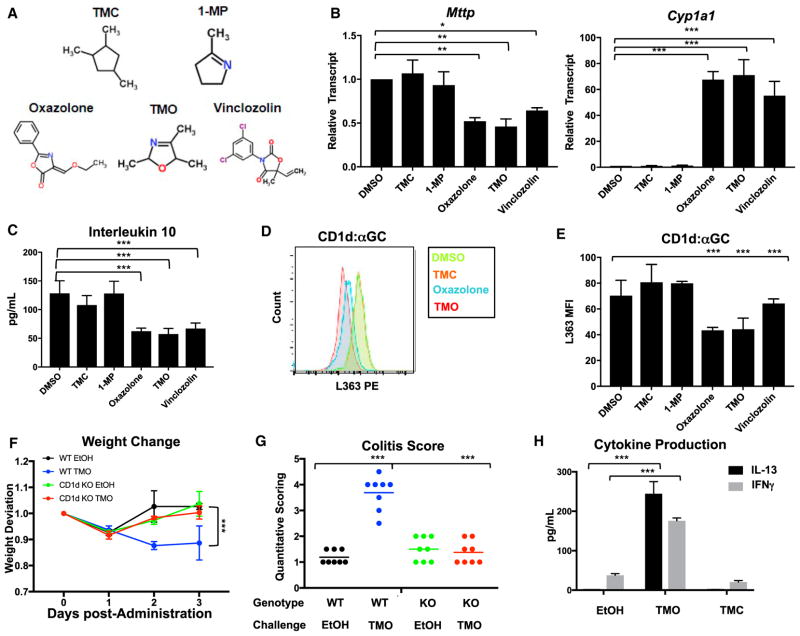
Figure 1. A Minimal Oxazole Structure Modulates Expression of Specific Gene Targets and Attenuates CD1d Restricted in Intestinal Epithelial Cells, Leading to Colonic Inflammation.
(A) Panel of oxazole-containing and control heterocylic natural and synthetic compounds.
(B) Relative transcript abundance in MODE-K cells stimulated with indicated compounds, normalized to β-actin. *p < 0.05, **p < 0.01, ***p < 0.001 (Student’s t test).
(C) IL-10 production in MODE-K cells conditioned with the indicated compounds, loaded with α-GC, followed by 24.7 iNKT hybridoma co-culture. ***p < 0.001 (Student’s t test).
(D and E) Representative traces and quantification of surface CD1d (D) loaded with α-GC (E) in MODE-K cells conditioned with indicated compounds.
(F) Weight change after intra-rectal administration of 1% TMO or EtOH (50% v/v) vehicle in WT or CD1d-deficient (KO) animals (n = 16–20). *p < 0.05 (Mann-Whitney U test)
(G) Quantitative scoring for colitis after intra-rectal administration of 1% TMO or ethanol (EtOH; 50% v/v) vehicle in WT or CD1d-deficient (KO) animals (n = 8).
(H) Quantification (ELISA) of Il-13 and IFN-γ production from colon explants 2 days after intra-rectal administration of 1% TMO, TMC, or EtOH (50% v/v) vehicle (n = 3). ***p < 0.001 (Student’s t test).
See also Figures S1 and S2 and Table S1.
MTP is a critical regulator of mucosal homeostasis that promotes epithelial barrier activity by controlling CD1d-restricted IL-10 production by epithelial cells (Colgan et al., 1999; Olszak et al., 2014). Thus, IEC-specific deletion of Mttp results in decreased CD1d-stimulated IL-10 production by the IEC, leading to the exaggerated inflammatory activity of hematopoietic cells in response to oxazolone challenge, resulting in increased colitis (Olszak et al., 2014). We modeled interactions between NKT cells and IECs using MODE-K cells and CD1d-restricted T cell hybridomas in a previously established CD1d-dependent co-culture system (van de Wal et al., 2003). These studies have shown that loading of CD1d with α-galactosyl ceramide (GC), a cognate lipid antigen, in this co-culture system leads to MTP-dependent, CD1d-restricted IL-10 production that is primarily derived from the IEC (Colgan et al., 1999; Olszak et al., 2014). Here, we found that MODE-K cells pre-conditioned with oxazolone exhibited attenuated IL-10 production in response to α-GC when placed in co-culture with an iNKT cell hybridoma (24.7), which specifically reacts to α-GC presented on CD1d (Figure 1C). Interestingly, epithelial-derived IL-10 was also suppressed when IECs stimulated with oxazolone were co-cultured with an autoreactive iNKT cell hybridoma (24.8) or an autoreactive non-invariant dNKT cell hybridoma (14S.6) in the absence of exogenously administered α-GC (Figures S1B and S1C). These effects were specific to the IEC, as co-culture of bone-marrow-derived dendritic cells with the 24.7 iNKT cell hybridoma and α-GC lead to production of IFN-γ (Figure S1D), but not IL-10 (data not shown), which was not affected by oxazolone or any of the oxazolone-related compounds described below.
In light of the decreased MTP expression observed in oxazolone-treated IECs (Figure 1B) and given that MTP loss alters the ability of CD1d to acquire exogenous antigens, such as α-GC, and CD1d-elicited IL-10 responses by epithelial cells (Dougan et al., 2005, 2007; Sagiv et al., 2007; Brozovic et al., 2004; Olszak et al., 2014), we investigated CD1d expression and lipid loading on oxazolone-conditioned IECs. IECs stimulated with oxazolone did not display altered CD1d protein expression or distribution between intracellular and cell surface pools (Figures S2A and S2B) but instead exhibited decreased loading of α-GC on CD1d at the cell surface (Figures 1D and 1E). These results were consistent with the decreased CD1d-restricted IL-10 protein production we observed in MODE-K:iNKT cell co-cultures (Figures 1C, S1B, and S1C) and suggest that oxazo-lone-induced alterations in MTP expression were associated with decreased CD1d function, affecting IL-10 production in response to iNKT cells.
Oxazolone is administered in experimental models of colitis and contact sensitivity but is also widely used in industry and pharmaceuticals as a pharmacophore for the generation of synthetic compounds, suggesting it may act as a potential environmental trigger of inflammation in sensitive hosts (Turchi, 2008). We therefore sought to identify a library of structural mimetics of oxazolone and test whether these molecules behaved in a similar manner. We performed a two-part in silico screen against three chemical databases of naturally occurring compounds (the Human Metabolome Database, the Super Natural II database, Dictionary of Natural Products) (Banerjee et al., 2015; Wishart et al., 2013), thereby limiting our search to compounds within a defined human enviroment. Natural compounds were queried in the standard simplified molecular input line entry system (SMILES) format, and Tanimoto similarities between oxazolone and test compounds were computed with the ChemMine Similarity Workbench, which calculated maximum common substructure (MCS) similarities with the Tanimoto coefficient. Further, Euclidean distance scores were calculated using a non-continuous atom-matching structural similarity function (NAMS) (Cao et al., 2008; Teixeira and Falcao, 2013). An initial screen identified 33 compounds with MCS > 0.5 and Euclidean distance scores cutoff of 0.9 (Table S1). Oxazolone consists of four major functional moieties: a five-membered oxazole ring, a 2′ phenylgroup, a 4’ ethoxymethylene substituent, and the 5′-carbonyl lactone. Our computational screen revealed the oxazole ring to be a distinctive and shared chemical substructure between the 33 compounds identified, all of which derived from dietary, microbial, and/or industrial sources. We then employed a second screen using the same parameters but instead substituting a minimal oxazole ring as our template structure. The analysis led to the identification of 63 naturally occurring oxazole-containing compounds (OxCs; including the 33 described above). Of particular interest, we noticed that many of these compounds derived from two major sources: diet and microbes. This led us to ask whether oxazolone is a molecular prototype for a broader class of environmental stimuli that feature an oxazole substructure capable of modulating epithelial CD1d-restricted responses in a cell-intrinsic manner.
To test this, we devised a systematic in vitro system to assess transcriptional and CD1d-restricted responses in IECs in response to stimulation with dietary OxC structures, including 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole (referred to as TMO; Figure 1A)—found in coffee, peanuts, and meats—that lacks the 2-phenyl ring, 5-ketone, and 4-ethoxymethylene present in oxazolone (Maga, 1978). Like oxazolone, TMO suppressed Mttp and induced cyp1a1 and Ido1 transcripts in IECs (Figures 1B and S1A). In contrast, two structurally related non-OxC heterocyclic aromatic compounds—1,2,4-trimethylcyclopentane (TMC; MCS = 0.5) and 2-methyl-1-pyrroline (1-MP; MCS = 0.83)—that substitute carbons at the 1’-oxygen and/or 3′-nitrogen did not elicit any transcriptional changes in the target genes assessed (Figure 1B). Moreover, IECs conditioned with oxazolone or TMO, but not with TMC or 1-MP, lead to decreased loading of α-GC on CD1d at the cell surface, thereby limiting CD1d-restricted IL-10 production in co-cultures with an iNKT hybridoma (24.7) (Figures 1C–1E). Overall, five of seven commercially available naturally occurring dietary OxCs, as well as vinclozolin (Figure 1A and Table S1), an OxC fungicide used in agriculture, phenocopied the transcriptional and CD1d-restricted epithelial responses observed after oxazolone stimulation.
A minimal Oxazole Structure Induces Colonic Inflammation in a CD1d-Dependent Manner
We next asked if a dietary-sourced minimal oxazole structure was sufficient to induce intestinal inflammation in vivo. Indeed, intra-rectal administration of TMO was observed to lead to increased weight loss and pathology characterized by superficial inflammation of the gut wall, neutrophil accumulation, and ulceration of the epithelial layer of the colon that was similar to the inflammation associated with oxazolone (Figures 1F, 1G, and S3A). Response to TMO, but not to TMC or vehicle, was characterized by production of Th2 and Th1 cytokines, including IL-13 and IFN-γ from colon explants (Figure 1H). Importantly, intestinal inflammation after TMO challenge was dependent on the presence of iNKT cells, as CD1d-deficient animals displayed no evidence of clinical symptoms or pathology (Figures 1F and 1G), as previously shown for oxazolone (Heller et al., 2002). In addition, host in vivo IEC responses to TMO, but not to TMC or vehicle, recapitulated the transcriptional changes observed in microarray and in vitro analyses, including downregulation of Mttp and induction of cyp1a1 (Figures S3B and S3C). Importantly, these studies were performed by direct administration of TMO to the colonic epithelium by rectal challenge without preceding skin sensitization, further suggesting that the OxC-induced effects observed were direct and did not require prior immune activation, as would be expected from a hapten-induced model. To confirm this, we compared the ability of oxazolone skin pre-sensitization to affect the responses to rectal challenge with TMO and vice versa (Figure S3D). These studies showed that pre-sensitization with oxazolone or TMO did not affect rectal responses to TMO or oxazolone, respectively; corroborating evidence that oxazolone or TMO was having direct effects on the colonic mucosa.
A Microbial-Derived Oxazole Structure Modulates Epithelial Responses and Leads to Intestinal Inflammation
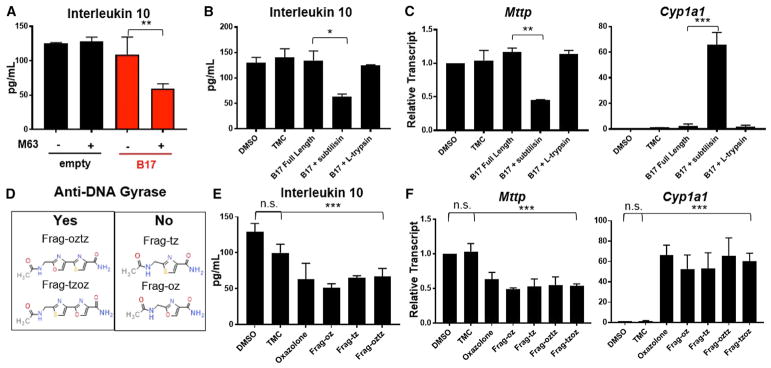
Our in silico analysis also identified a class of bacterial-derived toxins, featuring oxazole moieties with a high degree of similarity to a minimal oxazole template. These are termed thiazole-/oxa-zole-modified microcins (TOMMs) and make up a family of antimicrobial peptides featuring the presence of thiazol(in)e and oxazol(in)e heterocycles derived from cysteine, serine, and threonine residues on a ribosomally produced precursor peptide that confer a range of antimicrobial activities, including DNA gyr-ase inhibitors, translation inhibitors, and hemolytic toxins (Table S1) (Melby et al., 2011; Collin et al., 2013; Lee et al., 2008). Though chemically and functionally diverse, as a rule, TOMM biosynthetic gene clusters (BGCs) encode a leader peptide, enzymatic machinery including a cyclodehydratase and cyclodehydrogenase, that mediate post-translational installation of oxazole/thiazole moieties required for biological activity and, in some cases, an immunity gene that protects the host strain from the antimicrobial activities of the TOMM itself (Melby et al., 2011). Importantly, many TOMM BGCs have been described in a variety of human commensal communities as a means of providing host strains with ecological fitness advantages (Lee et al., 2008). Within the commensal populations, these BGC-encoded small-molecule bacterial signaling factors collectively have an enormous potential to mediate microbe-microbe interactions (Lee et al., 2008; Mazmanian et al., 2008; Sassone-Corsi et al., 2016). Therefore, we considered if microcins also regulated microbe-host interactions by modulating epithelial-derived CD1d immune responses in the host, similar to other OxCs. To address this question, we focused our attention on microcin B17 (MccB17, MCS = 0.75), originally isolated from strains of E. coli from the intestinal tract of newborns whose expression is linked to a plasmid (pMccB17) that carries the mcb operon, encoding the MccB17 toxin that acts as a DNA gyrase inhibitor (Table S1) (Li et al., 1996). The mcb operon was cloned into a pUC19 vector and transformed into a competent E. coli BSL1 human commensal strain (MG1655) (Collin et al., 2013). Bacteria transformed with mcb17 or empty plasmid was grown in Luria broth or M63 supplemented media to induce MccB17 synthesis (Collin et al., 2013), and bacterial lysates were incubated with IECs, after which we measured CD1d-restricted IL-10 production. We observed that lysates from bacterial isolates transformed with mcb and grown under MccB17 permissive conditions attenuated CD1d-restricted IL-10 production in MODE-K:iNKT cell co-cultures, whereas lysates isolated from bacteria grown under non-permissive conditions or transformed with empty plasmid had no effect (Figure 2A). To further examine whether suppression of CD1d-restricted responses was due to a MccB17 product, we purified full-length mature MccB17 by high-performance liquid chromatography (HPLC). To our surprise, purified MccB17 exerted no effects on CD1d-dependent IL-10 production (Figure 2B). Interestingly, although the full-length MccB17 microcin consists of a 3,093-Da peptide, several smaller heterocyclic mcb17-derived heterocyclic species, some of which retain DNA gyrase inhibitory activity, have been identified by matrix-assisted laser desorption/ionization time of flight (MALDI-ToF) mass spectrometry (MS) analysis. We hypothesized that the immunomodulatory effects of the MccB17-positive lysate might be a result of proteolytic and/or degradative products rather than the mature full-length MccB17 itself (Collin et al., 2013). B17 microcin is sensitive to cleavage by subtilisin, but not by trypsin (Asensio and Pérez-Díaz, 1976). Therefore, we digested purified full-length B17 with each protease and measured CD1d-restricted IL-10 responses in the IEC:iNKT cell co-culture assay to these proteo-lytic products (Figure 2B). In this case, cleavage products from subtilisin, but not from trypsin, were able to attenuate IL-10 production in IEC:iNKT co-cultures with α-GC. Similarly, we observed decreased Mttp and increased Cyp1a1 transcripts in MODE-K cells incubated with MccB17 subtilisin proteolytic products, but not with full-length B17 or MccB17 digested with trypsin (Figure 2C).
Figure 2. Microbial-Derived Oxazoles Modulate CD1d-Restricted Responses and Regulate Transcriptional Targets in Intestinal Epithelial Cells.
(A and B) IL-10 response in MODE-K cells pre-conditioned with vehicle (DMSO) or indicated compounds—(A) lysates from bacterial transformants (empty/ pUC19, B17) grown in Luria broth or M63 supplement and (B) purified MccB17 (B17) microcin and/or B17 microcin proteolytic digest (subtilisin, trypsin)—and subsequently loaded with α-GC followed by 24.7 iNKT hybridoma co-culture. *p < 0.05, **p < 0.01 (Student’s t test).
(C) Relative transcript abundance of target genes, Mttp and Ido1, in MODE-K cells to β-actin in MODE-K pre-conditioned as in (B). **p < 0.01, ***p < 0.001 (Student’s t test).
(D) Panel of synthetic analogs of B17 microcin proteolysis products with indicated anti-microbial activity.
(E) IL-10 response in MODE-K cells pre-conditioned with vehicle, oxazolone, or synthetic MccB17 microcin analogs and subsequently loaded with α-GC followed by 24.7 iNKT hybridoma co-culture. n.s., not significant; ***p < 0.001 (Student’s t test).
(F) Relative transcript abundance of target genes in MODE-K cells to β-actin in MODE-K pre-conditioned as in (E). n.s., not significant; ***p < 0.001 (Student’s t test).
Taken together, these results suggested that the ability of MccB17-derived toxins to affect epithelial transcriptional and CD1d-restricted responses is limited by the size of the product. To clarify this size restriction, we synthesized and screened a library of eight MccB17-derivative products with molecular weights (MWs) ranging from 183.17–857.82 Da whose anti-DNA gyrase activities have been previously described (Figure 2D) (Collin et al., 2013). Consistent with our hypothesis, four fragments, containing permutations of one or two oxazole/thiazole moieties, with MW < 270 Da were able to modulate CD1d-dependent IL-10 production and regulate Mttp and Cyp1a1, whereas synthetic products > 500Da were not (Figures 2E and 2F and data not shown). Importantly, synthetic analogs capable of exerting immunomodulatory effects were within the relative size range of both oxazolone (217.22 Da) and 2,4,5-trimethyl-2,5,-dihydro-1,3-oxazole (113.16 Da), but surprisingly, even synthetic products that did not confer anti-DNA gyrase activity were able to generate epithelial responses (Figures 2D–2F). Furthermore, both oxazole- and thiazole-containing compounds generated similar effects, consistent with their shared physicochemical properties (Figure 2D–2F).
A Synthetic B17 Microcin Derivative Induces Colonic Inflammation in a CD1d-Dependent Manner Independent of Antimicrobial Activity
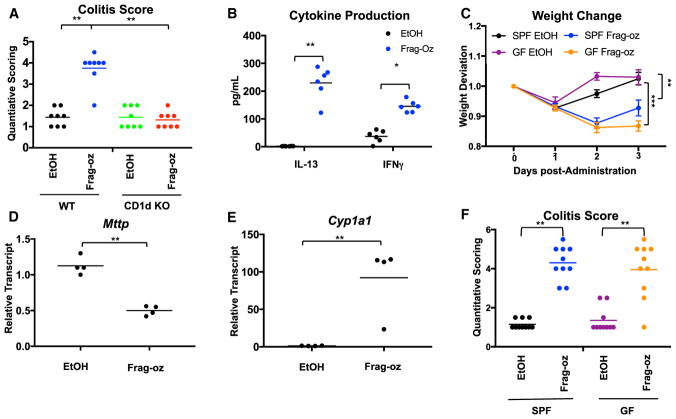
We next asked whether B17 microcin derivatives could induce intestinal inflammation in vivo. We therefore synthesized adequate quantities of a minimal B17 derivative (termed frag-oz; Figure 2D) to perform in vivo experiments and challenged animals via intra-rectal administration. Like with oxazolone and TMO, we observed CD1d-dependent pathology, as well as induction of both Th1 and Th2 cytokines from colon explants, suggesting that this molecule induces inflammation in an iNKT-dependent manner by comparing response in wild-type (WT) versus CD1d-deficient animals (CD1d knockout, KO) (Figures 3A–3C and S3A). We also observed decreased Mttp levels and induction of Cyp1a1 in IEC-enriched colonic fractions, suggesting that frag-oz exerts immunomodulatory effects via the epithelial compartment in vivo (Figures 3D and 3E). Though in vitro studies showed that frag-oz confers no anti-gyrase activity (Collin et al., 2013), to formally distinguish whether the intestinal inflammation observed was due to its antimicrobial properties or its direct effects on the host, we challenged GF and specific-pathogen-free (SPF) mice with frag-oz or vehicle. Consistent with the former, GF animals exhibited increased weight loss compared to animals raised under SPF conditions, together with severe inflammation as defined by quantitative histopathology (Figures 3C and 3F). These studies thus identify an oxazole-containing microbial mimic that promotes CD1d-dependent intestinal inflammation independent of its classical antimicrobial activity.
Figure 3. NKT Cells Are Required for Colonic Inflammation Induced by Frag-oz.
(A) Quantitative scoring for colitis after intra-rectal administration of 1% TMO or EtOH (50% v/v) vehicle in WT or CD1d-deficient (KO) littermates (n = 8). **p < 0.01 (Student’s t test).
(B) Quantification of IL-13 and IFN-γ production in overnatant of colon explants 2 days after intra-rectal administration of 1% TMO or EtOH (50% v/v) vehicle (n = 6). **p < 0.01 (Student’s t test).
(C) Weight change after intra-rectal administration of 1% Frag-oz or EtOH (50% v/v) vehicle in GF or SPF animals (n = 8). **p < 0.01, ***p < 0.001 (Mann-Whitney U test).
(D and E) Quantification of (D) Mttp and (E) Cyp1a1 transcripts (normalized to β-actin) from mucosal scraping 2 days after intra-rectal administration of 1% Frag-oz or EtOH (50% v/v) vehicle (n = 4). **p < 0.01, (Student’s t test)
(F) Quantitative scoring for colitis after intra-rectal administration of 1% TMO or EtOH (50% v/v) vehicle in GF or SPF animals (n = 8–9). **p < 0.01, p < 0.001 (Student’s t test).
See also Figure S3.
Activation of Epithelial AhR Pathways Exacerbates Response to Dietary and Microbial Oxazole Compounds by Limiting IL-10 Responses
The data presented support a model in which exposure to a broad class of oxazole (or thiazole) ring-containing compounds can direct transcriptional changes in responsive tissue compartments, such as the intestinal epithelium, that influence CD1d-restricted antigen presentation pathways, thereby augmenting iNKT (or non-invariant NKT) cell inflammatory responses, leading to colitis. These data also suggest the presence of cellular sensor(s) that are responsible for recognizing and transducing oxazole-dependent signals. Review of our previous microarray analyses identified two signature AhR targets, cyp1a1 and Ido1, involved in P450 and tryptophan metabolism, respectively, as inducible gene targets in the colonic epithelium (Olszak et al., 2014). Here, we demonstrate that oxazolone, vinclozolin, an industrial agent, and the dietary and microbial oxazole ligands, TMO and frag-oz, induced expression of these genes in vitro and in vivo. Interestingly, vinclozolin, an antifungal agent to which humans are exposed through agriculture and which affects CD1d-restricted pathways as an OxC as shown here, has been described as an AhR activator (Wambaugh et al., 2014).
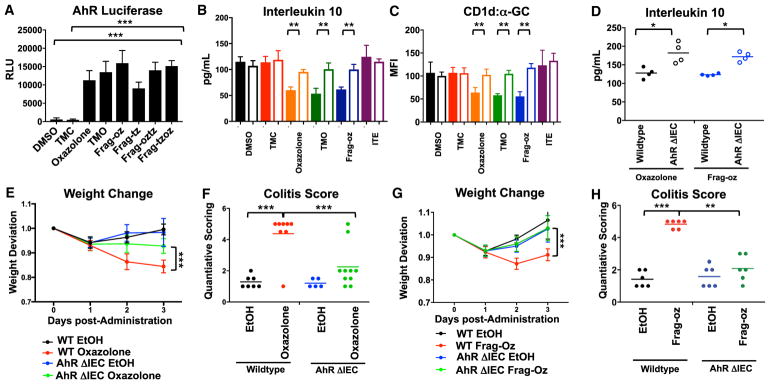
We therefore transfected IECs with an AhR firefly luciferase reporter and observed that oxazolone, TMO, and five additional dietary oxazoles, as well as four synthetic MccB17 derivatives, induced robust AhR transcriptional activity compared to vehicle or a non-oxazole heterocyclic control, TMC (Figure 4A). Consistent with this, small interfering RNA (siRNA)-mediated knockdown of AhR in MODE-K cells abrogated induction of cyp1a1 and IDO1 and partially restored Mttp expression (Figures S4A–S4C). Given that AhR regulates MTP expression, we asked whether this pathway could modulate epithelial-derived CD1d-restricted responses. Indeed, AhR deficiency caused by silencing AhR expression in epithelial cells reversed oxazolone-, TMO-, or frag-oz-mediated inhibition of CD1d-restricted IL-10 production in IEC:iNKT cell co-cultures via reduction of cell surface CD1d lipid antigen loading (Figures 4B and 4C). This was confirmed in hepatocytes obtained from Ahr−/− mice, which also exhibited resistance to the effects of oxazolone-, TMO-, frag-oz-, or vinclozolin-induced inhibition of IL-10 production in response to iNKT cells in the presence of α-GC (Figure S5). Interestingly, these effects were not attributed to general AhR function but instead reflected a specific response to oxazole-containing molecules, as a human cognate AhR ligand, 2-(1H-Indol-3-carbonyl)-4-thiazolecarboxylic acid methyl ester (ITE), did not modulate Mttp expression or CD1d-restricted responses (Figures 4B and 4C).
Figure 4. Activation of Aryl Hydrocarbon Receptor by Dietary and Microbial-Derived Oxazole Compounds.
(A) AhR promoter-reporter activity after MODE-K cells conditioned with indicated compounds. ***p < 0.001 (Student’s t test).
(B and C) MODE-K cells were transfected with scrambled (solid fill) or siRNA targeted against AhR (clear fill), conditioned with indicated compounds, and then loaded with α-GC followed by 24.7 iNKT hybridoma co-culture. Quantification of (B) CD1d-restricted IL-10 production. (C) Surface expressed CD1d loaded with α-GC. **p < 0.01, ***p < 0.001 (Student’s t test).
(D) Quantification of IL-10 from overnatant of colon explants 3 days after intra-rectal administration of 1% oxazolone or 1% Frag-oz EtOH (50% v/v) vehicle in WT or animals with AhR-deficiency in epithelial compartment (AhrΔIEC) (n = 4).
(E–H) (E and G) Weight change after intra-rectal administration of (E) 1% oxazolone or EtOH (50% v/v) vehicle or (G) 1% Frag-oz or EtOH (50% v/v) vehicle in WT or animals with AhR-deficiency in epithelial compartment (AhrΔIEC). ***p < 0.001 (Mann-Whitney U-test). (F and H) Quantitative scoring for colitis after intra-rectal administration of oxazolone (F) or frag-oz (H) in EtOH (50% v/v) in WT or animals with AhR-deficiency in epithelial compartment (AhrΔIEC) (n = 5). *p < 0.05; **p < 0.01; ***p < 0.001 (Student’s t test).
See also Figures S4 and S5.
These studies reveal a novel role for epithelial-derived AhR signals in promoting mucosal inflammation when exposed to OxCs. Consistent with this hypothesis, deletion of Ahr specifically in IECs (generated by crossing Ahrfl/fl to Villin-Cre transgenic animals, AhrΔIEC) resulted in elevated IL-10 production in colonic explants after oxazolone or frag-oz compared to WT controls (Figure 4D) and conferred host protection to oxazolone (Figures 4E and 4F) and frag-oz (Figures 4G and 4H) after intra-rectal challenge, as characterized by diminished weight loss and disease manifestations relative to that observed in Ahrfl/fl mice.
Oxazoles Induce the Generation of Tryptophan-Derived Metabolites that Attenuate Epithelial CD1d-Restricted Responses in an AhR-Dependent Manner
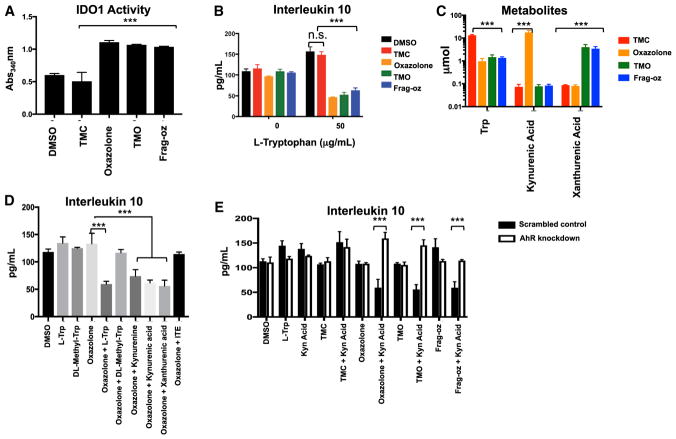
Our studies show that although AhR regulates CD1d-restricted responses to oxazole-containing compounds, this is not a property of all AhR ligands (such as ITE). This suggests that epithelial responses to OxCs generate AhR agonists uniquely capable of attenuating IL-10 production through CD1d-restricted mechanisms. It was therefore of interest that we observed IDO1 upregulation in IECs exposed to oxazoles, suggesting these compounds may increase IDO1 cellular activity (Figure S1A). Indeed, incubation of recombinant human IDO1 with oxazolone, TMO, or frag-oz enhanced the ability of IDO1 to convert tryptophan to formyl-kynurenine compared to control (TMC) or vehicle, indicating that oxazole compounds may function to enhance IDO1 enzymatic activity through direct mechanisms (Figure 5A). To test whether tryptophan metabolism is therefore involved in the AhR effects we observed, we cultured IECs in tryptophan-deficient media and found that CD1d-restricted IL-10 production was no longer attenuated by stimulation with oxazolone, TMO, or frag-oz when compared to TMC or vehicle controls (Figure 5B). Supplementation with exogenous L-tryptophan (L-Trp) restored responses to oxazolone, TMO, and frag-oz, resulting in attenuation of CD1d-restricted IL-10 production, supporting a role for tryptophan-derived metabolites in this process (Figure 5B). To specifically identify these tryptophan-derived products, we cultured IECs with deuterated L-trp (D5-Trp) and performed HPLC tandem mass spectrometry (HPLC-MS) on lysates stimulated with oxazolone, TMO, frag-oz, or TMC control (Figure 5C). We detected uptake of D5-Trp in TMC-stimulated IECs that was further metabolized to kynurenic acid and 3-hydroxy kynurenic acid (referred to as xanthurenic acid) only upon stimulation with oxazolone or TMO and frag-oz, respectively, within 6 hr of exposure to oxazoles (Figure 5C). To further investigate whether tryptophan-derived metabolites attenuate CD1d-restricted responses, we cultured IECs in tryptophan-deficient media supplemented with L-Trp, kynurenic acid, xanthurenic acid, or an upstream tryptophan product, kynurenine, in the presence or absence of oxazolone. Supplementation with exogenous tryptophan or its downstream products of catabolism restored IEC responses to oxazolone, as shown by reduced CD1d-restricted IL-10 production (Figure 5D). In contrast, supplementation with DL-methyl tryptophan (DL-Mtrp), a tryptophan competitive inhibitor of IDO1, did not restore suppression of CD1d-restricted IL-10 production in IECs cultured in tryptophan-deficient conditions and stimulated with oxazolone (Figure 5D). Finally, to ascertain whether these same metabolites identified in our LC-MS analyses functioned through the AhR pathway, we performed siRNA-mediated knockdown of AhR in IECs cultured under tryptophan-deficient conditions in the presence of exogenously administered kynurenic acid and measured CD1d-restricted IL-10 production after stimulation with OxCs (Figure 5E). Incubation of IECs with L-Trp or kynurenic acid alone, or in conjunction with TMC stimulation, had no effect on CD1d-restricted IL-10 production (Figures 5B and 5D). In contrast, IECs cultured under tryptophan-deficient conditions and stimulated with oxazolone, TMO, or frag-oz when supplemented with kynurenic acid led to decreased IL-10 production that was reduced when AhR was silenced (Figure 5E). Taken together, these latter studies are consistent with a model in which exposure of IECs to oxazole-containing compounds leads to the activation of IDO1 and generation of tryptophan-derived metabolites that induce the AhR pathway to downregulate CD1d-restricted responses associated with the production of IL-10 (Figure 6).
Figure 5. Oxazoles Induce the Generation of Tryptophan-Derived Metabolites that Attenuate Epithelial CD1d-Restricted Responses in an AhR-Dependent Manner.
(A) Recombinant IDO1 was incubated with L-tryptophan in the presence of oxazole (oxazolone, TMO, Frag-oz), control (TMC) compounds, or vehicle and formyl-kynurenine production measured via calorimetric assay. ***p < 0.001 (Student’s t test)
(B) MODE-K cells cultured with L-Trp-deficient media or media supplemented with exogenous L-Trp and CD1d-restricted IL-10 responses measured in MODE-K cells pre-conditioned with vehicle or indicated compounds. n.s., not significant; ***p < 0.001 (Student’s t test).
(C) HPLC-MS quantification of label-retaining metabolites in MODE-K cells grown under tryptophan-deficient conditions and supplemented with deuterated tryptophan and conditioned with the indicated compounds. ***p < 0.001 (Student’s t test)
(D) CD1d-restricted IL-10 responses in MODE-K cells pre-conditioned with vehicle or oxazolone in tryptophan-deficient media with supplementation with L-Trp, DL-Mtrp, kynurenine, kynurenic acid, or xanthurenic acid and then loaded with α-GC followed by 24.7 iNKT hybridoma co-culture. ***p < 0.001 (Student’s t test)
(E) CD1d-restricted IL-10 production by MODE-K cells transfected with scrambled (solid fill) or siRNA targeted against AhR (clear fill) in tryptophan-deficient media. CD1d-restricted IL-10 responses were measured following pre-conditioning with vehicle, TMC, oxazolone, TMO, or frag-oz with or without supplementation of L-Trp or kynurenic acid. ***p < 0.001 (Student’s t test).
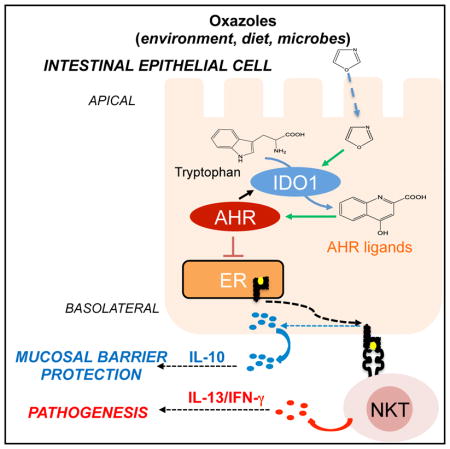
Figure 6. Exposure to Environmental Oxazoles (from Diet or Microbes) Influences CD1d-Restricted Responses in Intestinal Epithelial Cells through Production of Tryptophan Derivatives that Activate the Aryl Hydrocarbon Receptor Pathway.
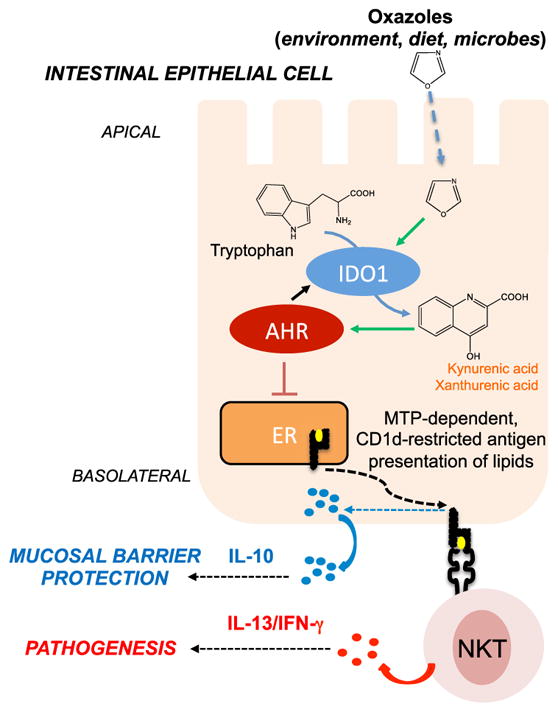
IECs promote mucosal barrier protection through microsomal triglyceride protein (MTP)-mediated CD1d-restricted production of IL-10 (blue circles). Upon exposure to oxazole compounds (derived from diet, microbes, or other environmental sources), these CD1d-restricted responses are attenuated through a mechanism involving the production of tryptophan-derived metab-olites (kynurenic acid, xanthurenic acid; orange squares) through the activity of IDO1 that trigger the AhR. Decreased, CD1d-restricted IL-10 production results in unrestrained inflammatory responses (IL-13/IFNγ, red dots) and resulting pathology. ER, endoplasmic reticulum.
DISCUSSION
In the studies described herein, we have used structural analyses of oxazolone, a compound previously shown to be capable of inducing inflammation of the colon (Boirivant et al., 1998, Heller et al., 2002), to identify a new and potentially broad class of environmental factors—derived from diet, microbes, and other sources—that we name OxCs. OxCs are capable of direct induction of intestinal inflammation through crosstalk of CD1d-restricted and AhR pathways in IECs and, together, suggest that these environmental agents are capable of activating CD1d-restricted pathways by phenocopying host responses to microbial factors.
Environmental and lifestyle factors have been identified as critical-risk elements for development of disorders of chronic inflammation, such as IBD (Molodecky and Kaplan, 2010). Consistent with this, chemical-induced models of intestinal inflammation have been crucial experimental tools for investigating the pathophysiology and the testing of therapeutic strategies for IBD (Wirtz et al., 2007; Kaser et al., 2010). Here, we have isolated the colitogenic activity within oxazolone to a five-membered heterocyclic oxazole moiety. Based on these observations, we inquired whether oxazolone is representative of a broader class of environmental factors that may be involved in the pathogenesis of IBD and possess a conserved or shared substructure. Oxazolone, first synthesized in 1883 by Erlenmeyer and Plöchl (Plöchl, 1884; Erlenmeyer, 1893), has been widely utilized as a synthetic building block for compounds with a range of pharmacological properties (Turchi, 2008) and is also naturally abundant in diet as a component of many thermally processed foods, pesticides, and other environmental sources (Maga, 1978). In addition, microbes are another abundant source of oxazoles as part of a structurally functionally diverse class of ribosomally derived peptides, dubbed TOMMs, that are widely disseminated across the phylogenetic spectra of bacterial secretion systems, including potential pathobionts associated with IBD pathogenesis or skin inflammation (Mazmanian et al., 2008; Melby et al., 2011). Together, this supports the notion that dietary and microbial-derived compounds with a common substructure, like oxazoles, can modulate host response by converging on conserved host immune cellular pathways (Figure 6).
We observed that exposure to select oxazole species modulates CD1d-restricted responses in epithelial cells in vitro that are characterized by downregulation of Mttp and reduced IL-10 production after CD1d crosslinking with NKT cell-associated receptors. This mechanism has been previously shown to reduce epithelial barrier activity in response to similar concentrations of oxazolone in the colon, leading to exacerbated inflammatory responses (Olszak et al., 2014). To what degree oxazole products accumulate within the intestinal tract and the physiological concentrations and circumstances required for triggering inflammatory responses are unknown. However, at least two of the structures we identified, TMO and vinclozolin, have been detected in human urine through studies associated with defining the human exposome (Table S1) (Wambaugh et al., 2014). Although not directly addressed in this study, it is interesting to consider whether accumulated exposure to oxazoles derived from multifactorial sources, including diet, microbes, industry, and/or agriculture, acts in an additive or combinatorial manner that includes other potential non-oxazole agents in modulating host responses that are associated with disease pathogenesis.
Our gene expression profiling of IECs exposed to oxazole compounds has identified a several genes (Figures 1B, 2C, 2F, 3E, S1A, and S4) involved in xenobiotic metabolism and tryptophan catabolism, including IDO1, a marker highly expressed in patients suffering from IBD, as well as in many animal models of colitis (Olszak et al., 2014; Ciorba, 2013; Wolf et al., 2004). Data presented here suggest that oxazole compounds directly enhance the ability of IDO1, the rate-limiting enzyme in tryptophan catabolism, to metabolize tryptophan (Figure 5A). This supports a role for the AhR as a potential cytosolic sensor or signal transducer of oxazole-induced products in the intestinal epithelium (Lanis et al., 2017). Indeed, specific depletion of the AhR in the epithelium was shown to alleviate the oxazole-induced suppression of CD1d-restricted anti-inflammatory responses, including production of IL-10 (Figure 4B). MS analyses identified at least two products of tryptophan catabolism, kynurenic acid and xanthurenic acid, that are specifically generated in IECs exposed to oxazolone or dietary and/or microbial oxazoles and their capacity to activate the AhR pathway in a manner that results in attenuation of CD1d-restricted IL-10 production. This observation is interesting, given that levels of tryptophan and its byproducts in the serum have provided a robust marker for gastrointestinal pathology in clinical settings (Ciorba, 2013).
In addition, we have shown that oxazoles also downregulate MTP, which is normally responsible for the loading of phospholipid antigens onto CD1d (Dougan et al., 2005, 2007). Consistent with our observations, altering MTP expression or activity has been previously shown to prevent transfer of phospholipids to CD1d or the ability of CD1d to acquire exogenous lipid antigens, such as α-GC (Dougan et al., 2005). Further, loss of MTP in IECs has been shown to disable the ability of iNKT cells to induce the barrier-protective cytokine, IL-10, by this cell type, as we have shown previously and demonstrated here (Olszak et al., 2014). Together, our data suggest that oxazoles alter presentation of lipid autoantigens (Figures S1B and S1C) and exogenous lipid antigens as shown with α-GC (Figures 1D, 1E, and 4C) to CD1d-restricted invariant and non-invariant NKT cells in a pathway that is dependent on AhR through the generation of tryptophan-derived metabolites. As both oxazolone and frag-oz induce inflammation in animals under GF conditions (Olszak et al., 2012) (Figures 3D and 3E), NKT-mediated responses to host-derived lipid antigens may be particularly important to oxazole-induced inflammation and further suggest that oxazoles may thus modulate autoimmune responses to self-lipid antigens.
Conversely, it is also interesting to consider whether microbes use oxazole products to target CD1d in order to establish their niche during primary colonization, potentially resulting in mucosal inflammation and pathology. We have previously demonstrated an important physiological role for CD1d during initial colonization of commensal and pathogenic bacteria (Nieuwenhuis et al., 2009). Consequently, generation of products that modulate CD1d activity, such as oxazoles, which are capable of specifically inhibiting IEC function, may affect the ability of microbes to colonize the intestine and potentially cause inflammation. While full-length MccB17 itself shared relatively low structural similarity with oxazolone, derivative products below 270 Da generated either by alkaline hydrolysis or proteolytic cleavage showed relatively high similarity with both oxazolone and TMO (Table S1). Importantly, we have observed that both the natural products and their synthetic analogs (Figure S6) display similar ability to attenuate CD1d-lipid antigen presentation and subsequent CD1d-restricted IL-10 production, as well as activate AhR responses specifically in IECs, resulting in inflammation associated with decreased epithelial-derived IL-10. That inflammation to MccB17 derivatives occurs in GF animals confirms that the effects of these compounds are directly impacting the host and not a result of their antimicrobial capacity. It is therefore intriguing to consider then that the accumulation of oxazole compounds derived from MccB17-producing and other TOMM-producing bacterial strains during a period of microbial competition may have a bystander effect on host immune responses, resulting in intestinal inflammation or other effects that benefit the organism at the expense of the host.
In conclusion, our studies illustrate a strategic approach for identifying potential triggers of inflammation by interrogating and evaluating structural mimics of lead compounds with established host responses and pathology. In this manner, by leveraging the knowledge that oxazolone acts as a colitogenic agent via modulation of CD1d-restricted pathways, we have identified OxCs as a new class of potential environmental agents with inflammation-inducing potential that are contained within a wide range of environmental sources, including diet and microbes. With respect to the latter, our studies further show that bacterial microcins, which are well known to play a role in microbe-microbe interactions, also possess important and previously unappreciated immune-regulating properties via their effects on CD1d-restricted pathways. Further, in both the case of environmental and microbial OxCs, we have found that they involve epithelial sensing by the AhR, which in turn adversely affects CD1d-restricted presentation of lipid antigens that are associated with barrier-protective production of IL-10. As a result, we also identified a novel pro-inflammatory role of AhR sensing that is associated with the intestinal epithelium and have brought forth the concept that environmental triggers of inflammation that are potentially associated with IBD may function in this manner by phenocopying the activity of microbial products with similar activity.
STAR ★ METHODS
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-mouse CD1d (1B1) FITC | BD Biosciences | Cat# 553845 |
| anti-mouse a-GalCer:CD1d complex PE | Biolegend | Cat# 140505 |
| anti-mouse aryl hydrocarbon receptor PE | BD Biosciences | Cat# 5657711 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dialyzed FBS | GIBCO | custom for R.S.B. Laboratory |
| Dialyzed RPMI | GIBCO | custom for R.S.B. Laboratory |
| Critical Commercial Assays | ||
| Mouse IL-13 DuoSet ELISA | R&D Systems | Cat# DY413 |
| Mouse IFN-γ ELISA MAX | Biolegend | Cat# 430801 |
| Mouse IL-10 ELISA Ready-Set-Go | eBioscience | Cat# 501125188 |
| IDO1 Inhibitor Screening Assay Kit | BPS Biosciences | Cat# 72021 |
| Luciferase Assay System | Promega | Cat# E4550 |
| Lipofectamine 2000 | Thermo Fisher Scientific | Cat# 11668019 |
| Intracellular Fixation and Permeablization Buffer Set | eBioscience | Cat# 88882400 |
| Omniscript RT Kit | QIAGEN | Cat# 205111 |
| SYBR Green I | Roche | Cat# 3003230001 |
| Oligonucleotides | ||
| 5′-GATGCTCCCCGGGCTGTATT-3′ | Mouse Bactin qPCR Forward Primer | R.S.B. Laboratory |
| 5′-GGGGTA CTTCAGGGTCAGGA-3′ | Mouse Bactin qPCR Reverse Primer | R.S.B. Laboratory |
| 5′-GAGAGCTGCAGGGCCCTTTGC-3′ | Mouse Interleukin 10 qPCR Forward Primer | R.S.B. Laboratory |
| 5′-CTCCCTGGTTTCTCTTCCCAAGACC-3′ | Mouse Interleukin 10 qPCR Reverse Primer | R.S.B. Laboratory |
| 5′-GCAGCCAGTACGCTCTTTTC-3′ | Mouse CD1d qPCR Forward Primer | R.S.B. Laboratory |
| 5′-ACAGCTTGTTTCTGGC AGGT-3′ | Mouse CD1d qPCR Reverse Primer | R.S.B. Laboratory |
| ′-GGACTTTTTGGATTTCAAAAGTGAC-3′ | Mouse Mttp qPCR Forward Primer | R.S.B. Laboratory |
| 5′-GGAGAAACGGTCATAATTGTG-3′ | Mouse Mttp qPCR Reverse Primer | R.S.B. Laboratory |
| 5′-TGGCACTCAGTAAAATATCTCCT-3′ | Mouse IDO1 qPCR Forward Primer | R.S.B. Laboratory |
| 5′CAGGCAGATTTCTAGCCACA-3′ | Mouse IDO1 qPCR Reverse Primer | R.S.B. Laboratory |
| 5′-GGCCACTTTGACCCTTACAA-3′ | Mouse Cyp1a1 qPCR Forward Primer | R.S.B. Laboratory |
| 5′-CAGGTAACGGAGGACAGGAA-3′ | Mouse Cyp1a1 qPCR Reverse Primer | R.S.B. Laboratory |
| mouse AhR siRNA | Thermo Fisher Scientific | Cat# 100360 |
| control siRNA | Thermo Fisher Scientific | Cat# AM4611 |
| Experimental Models: Cell Lines | ||
| MODE-K small intestinal epithelial cell line | Vidal et al., 1993 | R.S.B. Laboratory |
| 24.7 invariant Natural Killer T cell hybridoma | N/A | R.S.B. Laboratory |
| 24.8 invariant Natural Killer T cell hybridoma | N/A | R.S.B. Laboratory |
| 14S.6 non-invariant Naturall Killer T cell hybridoma | N/A | R.S.B. Laboratory |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J Specific Pathogen Free | The Jackson Laboratory | Cat# 000664 |
| CD1d 3/3 | N/A | R.S.B. Laboratory |
| Ahr tm3.1Bra/J | The Jackson Laboratory | Cat# 006203 |
| Villin-Cre | N/A | R.S.B. Laboratory |
| C57BL/6 Germ Free | Harvard Digestive Disease Center | R.S.B. Laboratory |
| AhR 3/3 | Taconic | Cat# 9166 |
| C57BL/6 | Taconic | Cat# B6 |
| Recombinant DNA | ||
| pGL4.43 Luc2pXRE | Promega | Cat# E4121 |
| pGL4.75 | Promega | Cat# E6931 |
| Renilla luciferase | Promega | Cat# E2261 |
| Software and Algorithms | ||
| Graphpad Prism 7.0 | GraphPad Sofware | https://www.graphpad.com/ |
| FloJo | FloJo, LLC and Illumina, Inc | https://www.flowjo.com/ |
| Other | ||
| Nikon Eclipse Ti Microscope | Nikon | N/A |
| BD FACS Aria II | BD Biosciences | N/A |
| MACSQuant Analyzer | Miltenyi Biotec | N/A |
| LTQ-XL Linear Ion Trap Mass Spectrometer | Thermo Fisher Scientific | N/A |
| CFX96 Real-Time System | Bio-Rad | N/A |
| 4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one | Sigma | Cat# 862207 |
| 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole | Sigma | Cat# CDS001470 |
| 2-methyl-1-pyrroline | Sigma | 381055 |
| Frag-oz | Collin et al., 2013 | A.L Laboratory |
| Frag-tz | Collin et al., 2013 | A.L. Laboratory |
| frag-oztz | Collin et al., 2013 | A.M. Laboratory |
| frag-tzoz | Collin et al., 2013 | A.M. Laboratory |
| Microcin B17 | Collin et al., 2013 | A.M. Laboratory |
| Vinclozolin | Sigma | Cat# 45705 |
| L-Tryptophan | Sigma | Cat# T0254 |
| DL-methyl tryptophan | Sigma | Cat# 860646 |
| L-trytophan-D5 | Sigma | Cat# sc-391262A |
| ITE | Tocris | Cat# 1803 |
| Subtilisin | Sigma | Cat# P5380 |
| Kynurenic-3,5,6,7,8-d5 | CDN Isotopes | Cat# D-4391 |
| L-Kynurenine | Sigma | Cat# K8625 |
| Kynurenic Acid | Sigma | Cat# K3375 |
| 4,8-dihydroxyquinoline-2-carboxylic acid | Sigma | Cat# D120804 |
| KRN7000 | Avanti Polar Lipids, Inc | Cat# 867000 |
| beta-glucosyl ceramide | Avanti Polar Lipids, Inc | Cat# 860547P |
| 1,2,4-trimethyl cyclopentane | Sigma | Cat# R289728 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Richard S. Blumberg (blumberg@bwh.harvard.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
Mice (C57BL/6J) were housed in a specific pathogen-free (SPF) barrier facility at Harvard Medical School: Wild-type, Ahrfl/fl, Villin-Cre, mice were purchased from The Jackson Laboratory. Ahr−/− mice were purchased from Taconic USA and maintained as heterozygotes. Cd1d1−/− Cd1d2−/− have been described. Animal studies were conducted in a gender and age matched (8–10 week) manner using littermates for each experiment, except for wild-type versus Cd1d−/− comparisons. SPF and Germ-free animals were bred and maintained in vinyl isolators in the Harvard Digestive Disease Center Gnotobiotic and Microbiology Core. For colitis experiments, mice were transferred to Optimice cages (Animal Care Systems) for daily monitoring under sterile conditions. All procedures were approved by the Harvard Medical Area Standing Committee on Animals.
Primary Cultures
Bone marrow derived cells (BMDC) were purified from mouse femurs and cultured for 7 days in RPMI (supplemented with 10% fetal bovine serum, 1% antibiotic-antimycotic, GIBCO and 20 ng/mL granulocyte-macrophage colon-stimulating factor, Biosource). For primary hepatocyte isolation, mice were anesthetized with intraperitoneal injection of ketamine (87 mg/kg body weight) plus xylazine (13 mg/kg body weight), after which the inferior vena cava was exposed, cannulated and perfused for 5 min with liver perfusion media (Invitrogen), followed by a 10 min perfusion with liver digestion media (Invitrogen). The digested liver was minced in hepatocyte was media (Invitrogen) and cells were pelleted and resuspended in Williams E medium (10% FBS, 10−7M dexamethasone, 10 μg/ml insulin and 5 μg/ml transferrin.
Bacterial Stocks and Purification
MccB17 was generated and purified as described (Collin et al., 2013; Li et al., 1996). Plasmid pUC19, wild-type or carrying the complete biosynthetic operon (mcbABCDEFG), was transformed into competent DH5α or MG1655 E. coli.
Synthesis of Microcin B17 and Derivatives
Overnight colonies were grown in LB media and used to inoculate M63 media (3 g/l KH2PO4, 7 g/l K2HPO4, 2 g/l (NH4)SO4, 1 mM MgSO4, 1 μg/ml thiamine, 0.2% glucose, 0.1 mg/ml ampicillin) or LB Media (0.1mg/mL ampicillin) and incubated for 48 h. Cells were pelleted and lysed in a boiling solution of 1 mM EDTA and 100 mM acetic acid. Cellular debris was removed by centrifugation, protein was quantified by BCA Assay kit (ThermoFisher Scientific) according to manufacturer’s instructions and lysates were serially titrated (1 μg-10 μg/mL) incubated with MODE-K cells for co-culture assays as described. Digestion of full length MccB17 ( Collin et al., 2013) by subtilisin (Sigma) (0.1 mM MccB17, 10% DMSO, 10 mM NaAcO, 5 mM Ca(AcO)2) or sequence grade trypsin (Promega) (0.5 mM 50 μg/mL trypsin, 1%DMSO in buffer (Promega) for 24 h to completion (Collin et al., 2013). Entire digested products (or equivalent buffer with enzyme) was then incubated with MODE-K cells for co-culture and qPCR analyses.
Synthesis of Frag-oz
Frag-oz, frag-tz, frag-oztz, and frag-tzoz originally were kindly provided by R. Payne, School of Chemistry University of Sydney or later synthesized by A.L. The compounds were synthesized according to established procedures for microcin B17-related oxazole and thiazole synthesis. Refer to Figure S6A. Step 1. Synthesis of 2-(aminomethyl)oxazole-4-carboxamide (Figure S6A) Tert-butyl ((4-carbamoyloxazol-2-yl)methyl)carbamate 11 (0.895 g, 3.71 mmol) was stirred in TFA/CH2Cl2 (1:1 v/v, 40 mL) at room temperature for 1 h. The solvent was removed under reduced pressure affording the title compound as a brown oil (0.367 g, 70%). 1H NMR (400 MHz, Methanol-d4) δ 8.42 (s, 1H), 4.28 (s, 2H). Step 2. 2-(acetamidomethyl)oxazole-4-carboxamide (Frag-oz): To a solution of 2-(aminomethyl)oxazole-4-carboxamide 2 (630mg, 4.46 mmol) in DMF (2.2 mL) at 0°C TEA (0.7 mL, 4.9 mmol) was added followed by Acetyl Chloride (0.35 mL, 4.9 mmol). The solution was stirred for 5 h at 0°C. The DMF was removed under reduced pressure and the crude product was purified by column chromatography on silica [DCM-Methanol, 9:1 (v/v)] affording the title compound as a white solid (400mg, 49%). 1H NMR (400 MHz, Methanol-d4) δ 8.30 (s, 1H), 4.48 (s, 2H), 1.99 (s, 3H). 13C NMR (101 MHz, Methanol-d4) δ 172.12, 163.71, 161.52, 142.01, 135.76, 35.87, 20.92.
Tert-butyl ((4-carbamoylthiazol-2-yl)methyl)carbamate (4)
Aqueous ammonia (28% w/w, 3,1 mL, 40 mmol) was added to a solution of ethyl 2-(((tert-butoxycarbonyl)amino)methyl)thiazole-4-carboxylate2 3 (381 mg, 1.33 mmol) in methanol (1,3 mL). After 16 h at 45°C, the reaction mixture was concentrated affording the title compound as a brown oil (315mg, 92%) which was used directñy in the next step. 1H NMR (400 MHz, Methanol-d4) δ 8.10 (s, 1H), 4.49 (s, 2H), 1.45 (s, 9H).
2-(aminomethyl)thiazole-4-carboxamide (5)
Tert-butyl ((4-carbamoylthiazol-2-yl)methyl)carbamate 4 (0.342 g, 1.331 mmol) was stirred in TFA/CH2Cl2 (1:1 v/v, 13 mL) at room temperature for 1.5 h. The solvent was removed under reduced pressure affording the title compound as a green oil (0.150, 72%) which was used directly in the next step. 1H NMR (400 MHz, Methanol-d4) δ 8.25 (s, 1H), 4.41 (s, 2H).
Synthesis of Frag-tz 2-(acetamidomethyl)thiazole-4-carboxamide
Refer to Figure S6B. To a solution of 2-(aminomethyl)thiazole-4-carboxamide 5 (204mg, 1.30 mmol) in DMF (6.5mL) at 0°C, Triethylamine (TEA, 0.2 mL, 1.43 mmol) was added followed by Acetyl Chloride (0.1 mL, 1.43 mM). The solution was stirred for 5 h at 0°C. The solvent was removed under reduced pressure and the crude product was purified by column chromatography on silica [CH2Cl2 /Methanol, 9:1 (v/v)] affording the title compound as a white solid (80mg, 31%). 1H NMR (400 MHz, Methanol-d4) δ 8.11 (s, 1H), 4.63 (s, 2H), 2.01 (s, 3H). 13C NMR (101 MHz, Methanol-d4) δ 172.13, 169.43, 164.11, 149.10, 124.41, 40.47, 21.01.
METHOD DETAILS
Experimental colitis model
For reciprocal sensitization studies (Figure S3), mice were pre-sensitized by epicutaneous application of 3% w/v oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one, Sigma-Aldrich) or 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole (Sigma-Aldrich) in 100% ethanol (200 μL volume). Five days later, animals were re-challenged intra-rectally (through a 3.5F catheter) with 1% vehicle, oxazolone or 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole in 50% ethanol (5 μL/g of body weight) as indicated. For all other studies, vehicle, oxazolone, 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole, 1,2,4-trimethylcyclopentane (Sigma-Aldrich) were administered intrarectally (through a 3.5F catheter) as a 1% solution in 50% ethanol (5 μl/g of body weight). Body weight, rectal bleeding, and stool consistency were analyzed 1-2 times daily. Tissues obtained at the indicated time points were embedded in paraffin, stained with hematoxylin and eosin, and examined by a pathologist (Dr. Jon Glickman) in a blinded fashion for evidence of colitis according to five established criteria: mononuclear inflammation, crypt hyperplasia, epithelial injury, neutrophilic inflammation, and hypervascularization grading on 4 point scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe).
Colon organ culture
Standardized segments (1 cm × 1 cm) of the transverse colon were washed in cold PBS supplemented with penicillin and streptomycin (GIBCO) and cultured in 24-well flat-bottom culture plates (Falcon) in RPMI 1640 media (GIBCO) for 24 h at 37°C. Supernatants were analyzed for cytokines IL-10, IL-13 and IL-1β by ELISA (all BD).
Cells and Antigen presentation assays
1 × 105 BMDCs, primary hepatocytes or 0.5 × 105 MODE-K cells (Vidal et al., 1993) were incubated in 96 well plates (Corning) maintained in RPMI (supplemented with 2% fetal bovine serum, 1% antibiotic-antimycotic, GIBCO) were pulsed with the indicated compounds dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 μM for 36–48h. Cells were washed three times with sterile phosphate buffered saline (PBS, GIBCO) after which cells were incubated with 100 ng ml−1 alpha-galactosyl ceramide/KRN7000 or beta-glucosyl ceramide (Avanti Polar Lipids) for 3h, washed three times with sterile PBS, before addition of 2 × 104 NKT hybridoma cells including 24.7, 24.8, and 14S6 overnight co-culture after which supernatant was collected and clarified by centrifugation. Mouse IL-10, IFNγ production was assessed by ELISA (OptEIA, BD Biosciences). For aryl hydrocarbon receptor siRNA mediated knockdown, MODE-K cells were transfected with AM100441, AM 100360, or Silencer negative control siRNAs (ThermoFisher Scientific) using Lipofectamine 2000 (Life Technologies) according to manufacturer’s instructions. AhR silencing was verified 48h after transfection by quantitative polymerase chain reaction.
Identification of Tryptophan Derived Metabolites
MODE-K cells were cultured in dialyzed FBS and RPMI (GIBCO #26400044, RPMI 1640 cGMP #100001229, all amino acids < 1nM,) and supplemented with essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine at 1 μM) and supplemented with deuterated tryptophan (1 μM D5-Trp, Santa Cruz sc-391262, see Key Resources Table). Lysates were extracted from cell pellets in 80% Methanol and analyzed with reverse phase HPLC-MS (Agilent C18 column connected with Thermo Scientific LTQ-XL, see Key Resources Table).
IDO1 enzymatic assay
Recombinant His-tagged IDO1 was incubated with 10nM concentrations of TMC, oxazolone, TMO, or frag-oz and assay was performed according to the manufacturer’s instruction (BPS Bioscience, San Diego, CA, see Key Resources Table.)
CD1d and CD1d-lipid complex measurements by flow cytometry
MODE-K cells were pulsed with 10 μM indicated compounds dissolved in DMSO for 36–48h. Cells were washed three times with sterile PBS after which cells were incubated with 100 ng ml−1 alpha-galactosyl ceramide/KRN7000 or the non-agonistic compound, beta-glucosyl ceramide (data not shown), for 3 h, washed three times with sterile PBS. Cells were lifted in 10 mM EDTA/PBS and stained with the following antibodies: mouse L363 (eBiosciences, see Key Resources Table), mouse CD1d (BD Bioscience, see Key Resources Table). For intracellular staining, cells were fixed using the Intracellular Fixation and Permeabilization Buffer Set (eBioscience, see Key Resources Table) according to the manufacturer’s instructions. Flow cytometry was performed on MACSQuant Analyzer (Miltenyi Biotec) and analyzed using FlowJo software.
Quantitative polymerase chain reaction assays
RNA samples were prepared using an RNeasy Mini Kit and cDNAs were synthesized using the Omniscript RT Kit (QIAGEN, see Key Resources Table). Real-time RT–PCR was performed using SYBR Green I Master Mix (Roche) and a CFX96 Real-Time System (Bio-Rad, see Key Resources Table). Values were normalized to β-actin (see Key Resources Table).
Luciferase promoter-reporter assays
For aryl hydrocarbon receptor luciferase assays, MODE-K cells were cultured on 6-well plates (Corning). pGL4.43 Luc2pXRE (Promega) or empty pGL4.43 (Promega) and 100 ng Renilla luciferase (Promega) was transfected into MODE-K cells using Lipofectamine 2000. Twenty four hours later, MODE-K cells were conditioned with indicated compounds for 48 h. Cells were lysed and firefly luciferase (normalized to renilla luciferase) was measured using Dual Luciferase Reporter Assay System (Promega)
In-silica similarity screening
We used two-step in-silico screening process. First, broad similarity screening was performed to identify oxazole related natural compounds from various databases including Pubchem, Super Natural II a database of natural products, Dictionary of natural products Chemnet database, and The Human Metabolome Database (Wishart et al., 2013) (Table S1). Second, focused screen search was performed based on the results of first screening to identify natural compounds related to a 5-membered oxazole ring of oxazolone with emphasis to find minimal structural features of oxazolone for its inflammatory activity. This focused screen selection was based on identification of natural compounds with Tanimoto similarity of more than 0.5 and/or Euclidean distance scores cutoff of 0.9 (Table S1). Natural compounds were queried in the standard SMILES format. Tanimoto similarities between compounds were computed with the Chemmine Similarity Workbench, which calculated maximum common substructure (MCS) similarities with the Tanimoto coefficient (Cao et al., 2008). Euclidean distance scores were calculated using Noncontiguous atom matching structural similarity function (NAMS) (Teixeira and Falcao, 2013). The following formulas were used to calculate MCS similarity and TS score of natural compounds with Oxazolone and Oxazole ring substituted with a methyl group at the 2, 4 and 5 position. MCS similarity score: Atom number (AN) in MCS/AN in smaller molecule, TS score: AN in MCS/ (AN in smaller molecule + AN in larger molecule –AN in MCS). Based on the results of the screening process, TMO with Tanimoto/Euclidean similarity score of 0.78/0.97 was selected as one of the natural compound for in vivo experiments. To further investigate the role of nitrogen and oxygen atoms of the oxazole ring and correlate them with colitogenic activity, Trimethylcyclopentane (TMC) and 2-Methyl-1-pyrroline (1MP) were selected as additional controls. In addition, Vinclozolin was selected based on Euclidean distance score of 0.94 and widespread fungicide usage.
Supplementary Material
Figure S1. A Minimal Oxazole Structure Modulates Intestinal Epithelial Transcriptional and Antigen Presentation Capacity to Invariant and Non-variant NKT Cells, Related to Figure 1
(A) Relative transcript abundance in MODE-K cells conditioned with the indicated compounds, normalized to β-actin. **p < 0.01 (Student’s t test).
(B and C) CD1d-restricted Interleukin 10 production in the absence of exogenous lipid supplementation in MODE-K cells conditioned with the indicated compounds followed by co-culture with (B) invariant NKT (24.8) or (C) non-invariant NKT (14S.6) hybridomas. ***p < 0.001 (Student’s t test).
(D) Interferon γ production in bone marrow derived dendritic cells conditioned with the indicated compounds followed by loading with α-galactosyl ceramide and co-cultured with 24.7 iNKT hybridoma.
Figure S2. A minimal Oxazole Structure Does Not Alter CD1d Protein Expression or Cellular Distribution, Related to Figure 1
(A and B) Quantification of surface and intra-cellular CD1d protein expression in MODE-K cells conditioned with the indicated compounds for 48hr, as measured by (A) flow cytometry and (B) relative proportions of intracellular and cell surface CD1d protein abundance.
Figure S3. Intestinal Inflammation, Epithelial Response, and Pathology to Oxazole-Containing Compounds Does Not Require Apriori Host Sensitization, Related to Figure 3
(A) Representative sections of wild-type animals administered 1% of the indicated compounds (50% EtOH v/v) by intra-rectal challenge and analyzed by H&E stain after 3 days. 10X magnification, scale bar (40 μM).
(B and C) Quantification of B. Mttp and C. Cyp1a1 transcripts (normalized to β-actin) from mucosal scrapings 2 days after intra-rectal challenge with 1% TMO, 1% TMC or EtOH (50%v/v) vehicle (n = 3). n.s. not significant, *p < 0.05, ***p < 0.001 (Student’s t test).
(D) Animals were sensitized by topical application of the indicated compounds followed by intra-rectal administration of 1% TMO, 1% oxazolone, or EtOH (50% v/v) vehicle in wild-type C57BL/6 animals. Quantitative colitis scoring was assessed on colons harvested 3 days after intra-rectal challenge. ***p < 0.001 (Student’s t test).
Figure S4. Related to Figure 4
(A–C) Aryl hydrocarbon receptor mediates expression of a subset of gene targets in response to oxazole containing compounds: MODE-K cells transfected with control or AhR specific siRNA, conditioned with the indicated compounds and relative transcript abundance was measured normalized to β-actin. (A) cyp1a1, (B) Ido1, (C) Mttp n.s. not significant, ***p < 0.001 (Student’s t test).
Figure S5. Aryl Hydrocarbon Receptor Attenuates CD1d-Restricted Responses in Primary Hepatocytes, Related to Figure 4
Interleukin 10 production from primary hepatocytes derived from WT or AhR-deficient (KO) animals were conditioned with the indicated compounds, loaded with α-galactosyl ceramide followed by co-culture with 24.7 iNKT hybridoma. ***p < 0.001 (Student’s t test).
Figure S6. Synthesis of Frag-oz and Frag-tz, Related to STAR Methods
(A and B) A schematic outline for synthesis of MccB17 derived products (A) Frag-oz. (B) Frag-tz.
Highlights.
Oxazoles derived from diet, industrial sources, and microbes activate IDO1
Environmental oxazoles induce tryptophan-derived metabolites to activate AhR in IECs
AhR activation in IECs limits CD1d-restricted production of IL-10
Oxazole activation of AhR in IECs results in iNKT-mediated intestinal inflammation
Acknowledgments
R.S.B. is supported by NIH grants DK044319, DK053056, DK051362, DK088199, and 5P01AI073748 and the Harvard Digestive Diseases Center (P30DK034854). S.S.I. and T.G. are supported by the Crohn’s and Colitis Foundation of America Research Fellow Awards (383527 and 418509). S.F.O is supported by NIH grant DK102771. We thank Drs. Roberto Kolter and Dennis Kasper (Harvard Medical School) for reagents and discussion of data, Drs. Keith Houck and Jill Franzosa (Environmental Protection Agency) for helpful discussions and Toxcast21 data, and Vladimir Yeliseyev and Marla Marquez for technical assistance. We thank Amanjot Riar and Blumberg laboratory members for assistance in manuscript preparation. A.M.’s lab is funded by the Biotechnology and Biosciences Research Council (UK) Institute Strategic Programme Grants BB/J004561/1 and BB/P012523/1.
Footnotes
Supplemental Information includes six figures and one table and can be found with this article online at https://doi.org/10.1016/j.cell.2018.04.037.
AUTHOR CONTRIBUTIONS
Conceptualization, S.S.I., T.G., and R.S.B.; Methodology, S.S.I., T.G., A.G., S.F.O., F.C., C.S., A.L., A.M., J.F.N., R.L., and R.S.B.; Analysis, S.S.I., T.G., A.G., C.S., F.C., A.L., A.M., J.G., and R.S.B.; Writing and Editing S.S.I., T.G., A.G., P.S.A.d.S., R.B.S., G.B., R.H., A.L., A.M., J.F.N., R.B.S., and R.S.B.
DECLARATION OF INTERESTS
The authors declare no competing interests.
References
- An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio C, Pérez-Díaz JC. A new family of low molecular weight antibiotics from enterobacteria. Biochem Biophys Res Commun. 1976;69:7–14. doi: 10.1016/s0006-291x(76)80264-1. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Erehman J, Gohlke BO, Wilhelm T, Preissner R, Dunkel M. Super Natural II–a database of natural products. Nucleic Acids Res. 2015;43:D935–D939. doi: 10.1093/nar/gku886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- Cao Y, Jiang T, Girke T. A maximum common substructure-based algorithm for searching and predicting drug-like compounds. Bioinformatics. 2008;24:i366–i374. doi: 10.1093/bioinformatics/btn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–152. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Hershberg RM, Furuta GT, Blumberg RS. Ligation of intestinal epithelial CD1d induces bioactive IL-10: critical role of the cytoplasmic tail in autocrine signaling. Proc Natl Acad Sci USA. 1999;96:13938–13943. doi: 10.1073/pnas.96.24.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin F, Thompson RE, Jolliffe KA, Payne RJ, Maxwell A. Fragments of the bacterial toxin microcin B17 as gyrase poisons. PLoS ONE. 2013;8:e61459. doi: 10.1371/journal.pone.0061459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer E. Ueber die Condensation der Hippursäure mit Phtalsäureanhydrid und mit Benzaoldehyd. Justus Liebigs Ann Chem. 1893;275:1–8. [Google Scholar]
- Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, Rieder F, Scaldaferri F, Schirbel A, Scarpa M, et al. IL-13Rα2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63:1728–1736. doi: 10.1136/gutjnl-2013-305671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbachev AV, Fairchild RL. Induction and regulation of T-cell priming for contact hypersensitivity. Crit Rev Immunol. 2001;21:451–472. [PubMed] [Google Scholar]
- Grose RH, Thompson FM, Baxter AG, Pellicci DG, Cummins AG. Deficiency of invariant NK T cells in Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2007;52:1415–1422. doi: 10.1007/s10620-006-9261-7. [DOI] [PubMed] [Google Scholar]
- Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, Gerich ME, Glover LE, Kominsky DJ, Colgan SP. Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. 2017;10:1133–1144. doi: 10.1038/mi.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Mitchell DA, Markley AL, Hensler ME, Gonzalez D, Wohlrab A, Dorrestein PC, Nizet V, Dixon JE. Discovery of a widely distributed toxin biosynthetic gene cluster. Proc Natl Acad Sci USA. 2008;105:5879–5884. doi: 10.1073/pnas.0801338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Milne JC, Madison LL, Kolter R, Walsh CT. From peptide precursors to oxazole and thiazole-containing peptide antibiotics: microcin B17 synthase. Science. 1996;274:1188–1193. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- Maga JA. Oxazoles and oxazolines in foods. J Agric Food Chem. 1978;26:1049–1050. doi: 10.1021/jf60219a030. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Melby JO, Nard NJ, Mitchell DA. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr Opin Chem Biol. 2011;15:369–378. doi: 10.1016/j.cbpa.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6:339–346. [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis EE, Matsumoto T, Exley M, Schleipman RA, Glickman J, Bailey DT, Corazza N, Colgan SP, Onderdonk AB, Blumberg RS. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, Brugman S, Yamaguchi K, Ishikawa H, Aiba Y, et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–1250. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin CS, Jobin C, Brand S, Sotlar K, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plöchl J. Uber einige Derivate der Benzoylmidozimtsaure. Ber Dtsch Chem Ges. 1884;17:1616–1624. [Google Scholar]
- Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv Y, Bai L, Wei DG, Agami R, Savage PB, Teyton L, Bendelac A. A distal effect of microsomal triglyceride transfer protein deficiency on the lysosomal recycling of CD1d. J Exp Med. 2007;204:921–928. doi: 10.1084/jem.20061568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi M, Nuccio SP, Liu H, Hernandez D, Vu CT, Takahashi AA, Edwards RA, Raffatellu M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540:280–283. doi: 10.1038/nature20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton H, Popple A, Gellatly N, Maxwell G, Williams J, Friedmann PS, Kimber I, Dearman RJ. Anti-hapten antibodies in response to skin sensitization. Contact Dermat. 2016;74:197–204. doi: 10.1111/cod.12486. [DOI] [PubMed] [Google Scholar]
- Teixeira AL, Falcao AO. Noncontiguous atom matching structural similarity function. J Chem Inf Model. 2013;53:2511–2524. doi: 10.1021/ci400324u. [DOI] [PubMed] [Google Scholar]
- Turchi IJ. Chemistry of Heterocyclic Compounds. John Wiley & Sons; 2008. [Google Scholar]
- van de Wal Y, Corazza N, Allez M, Mayer LF, Iijima H, Ryan M, Cornwall S, Kaiserlian D, Hershberg R, Koezuka Y, et al. Delineation of a CD1d-restricted antigen presentation pathway associated with human and mouse intestinal epithelial cells. Gastroenterology. 2003;124:1420–1431. doi: 10.1016/s0016-5085(03)00219-1. [DOI] [PubMed] [Google Scholar]
- van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, Polman CH, Rustemeyer T, Lips P, van den Eertwegh AJ, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- Vidal K, Grosjean I, evillard JP, Gespach C, Kaiserlian D. Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J Immunol Methods. 1993;166:63–73. doi: 10.1016/0022-1759(93)90329-6. [DOI] [PubMed] [Google Scholar]
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ Sci Technol. 2014;48:12760–12767. doi: 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sedimbi S, Löfbom L, Singh AK, Porcelli SA, Cardell SL. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2018;11:131–143. doi: 10.1038/mi.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, Sonnenburg JL, Comstock LE, Bluestone JA, Fischbach MA. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013;11:e1001610. doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, et al. HMDB 3.0–The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A Minimal Oxazole Structure Modulates Intestinal Epithelial Transcriptional and Antigen Presentation Capacity to Invariant and Non-variant NKT Cells, Related to Figure 1
(A) Relative transcript abundance in MODE-K cells conditioned with the indicated compounds, normalized to β-actin. **p < 0.01 (Student’s t test).
(B and C) CD1d-restricted Interleukin 10 production in the absence of exogenous lipid supplementation in MODE-K cells conditioned with the indicated compounds followed by co-culture with (B) invariant NKT (24.8) or (C) non-invariant NKT (14S.6) hybridomas. ***p < 0.001 (Student’s t test).
(D) Interferon γ production in bone marrow derived dendritic cells conditioned with the indicated compounds followed by loading with α-galactosyl ceramide and co-cultured with 24.7 iNKT hybridoma.
Figure S2. A minimal Oxazole Structure Does Not Alter CD1d Protein Expression or Cellular Distribution, Related to Figure 1
(A and B) Quantification of surface and intra-cellular CD1d protein expression in MODE-K cells conditioned with the indicated compounds for 48hr, as measured by (A) flow cytometry and (B) relative proportions of intracellular and cell surface CD1d protein abundance.
Figure S3. Intestinal Inflammation, Epithelial Response, and Pathology to Oxazole-Containing Compounds Does Not Require Apriori Host Sensitization, Related to Figure 3
(A) Representative sections of wild-type animals administered 1% of the indicated compounds (50% EtOH v/v) by intra-rectal challenge and analyzed by H&E stain after 3 days. 10X magnification, scale bar (40 μM).
(B and C) Quantification of B. Mttp and C. Cyp1a1 transcripts (normalized to β-actin) from mucosal scrapings 2 days after intra-rectal challenge with 1% TMO, 1% TMC or EtOH (50%v/v) vehicle (n = 3). n.s. not significant, *p < 0.05, ***p < 0.001 (Student’s t test).
(D) Animals were sensitized by topical application of the indicated compounds followed by intra-rectal administration of 1% TMO, 1% oxazolone, or EtOH (50% v/v) vehicle in wild-type C57BL/6 animals. Quantitative colitis scoring was assessed on colons harvested 3 days after intra-rectal challenge. ***p < 0.001 (Student’s t test).
Figure S4. Related to Figure 4
(A–C) Aryl hydrocarbon receptor mediates expression of a subset of gene targets in response to oxazole containing compounds: MODE-K cells transfected with control or AhR specific siRNA, conditioned with the indicated compounds and relative transcript abundance was measured normalized to β-actin. (A) cyp1a1, (B) Ido1, (C) Mttp n.s. not significant, ***p < 0.001 (Student’s t test).
Figure S5. Aryl Hydrocarbon Receptor Attenuates CD1d-Restricted Responses in Primary Hepatocytes, Related to Figure 4
Interleukin 10 production from primary hepatocytes derived from WT or AhR-deficient (KO) animals were conditioned with the indicated compounds, loaded with α-galactosyl ceramide followed by co-culture with 24.7 iNKT hybridoma. ***p < 0.001 (Student’s t test).
Figure S6. Synthesis of Frag-oz and Frag-tz, Related to STAR Methods
(A and B) A schematic outline for synthesis of MccB17 derived products (A) Frag-oz. (B) Frag-tz.