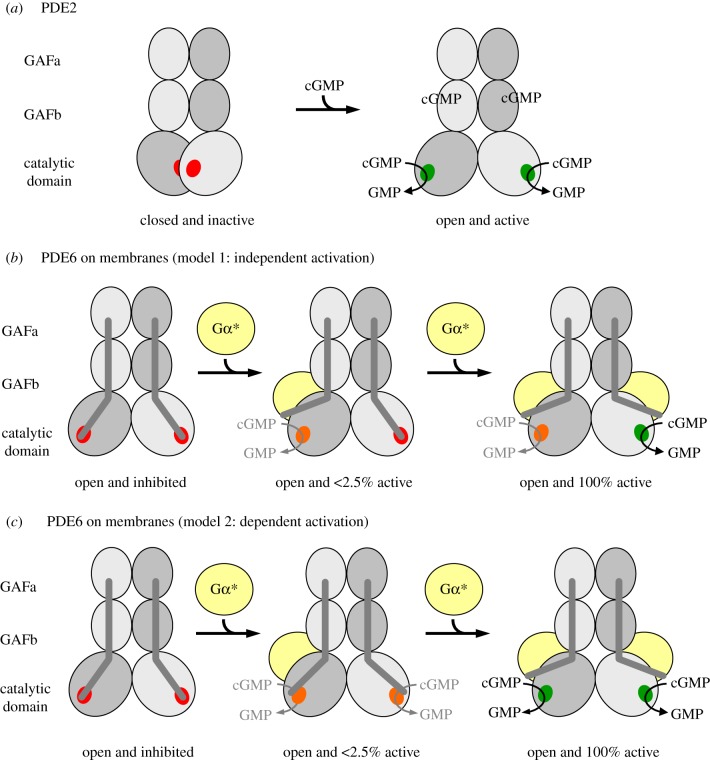
Figure 5.
Models of PDE activation. Each catalytic PDE subunit consists of two GAF domains and one catalytic domain. The three domains of each catalytic subunit are arranged in a crossover architecture (see figure 4a and electronic supplementary material, appendix for details). (a) Activation of PDE2 as proposed by Pandit et al. [19]: in the closed, inactive PDE2 conformation, access of the substrate to the catalytic cGMP-binding sites (red) is blocked by mutual inhibition of the catalytic domains. Cooperative binding of cGMP to the GAFb domains induces outward rotation of the catalytic domains and hence formation of the open, active PDE2 conformation. (b,c) Activation of membrane-associated rod PDE6: the PDE6αβ-dimer adopts an open conformation, which is inhibited by tight interaction with two PDE6γ subunits (dark-grey rods) in the resting state. Activation of membrane-associated PDE6 is due to removal of inhibition following the successive binding of two Gα*s (membrane is omitted for clarity). The catalytic cGMP-binding sites are either fully inhibited (red), or have a very low (less than 2.5% of maximum; orange) or high hydrolytic cGMP activity (green). In model 1 (b), the two catalytic PDE6 subunits are intrinsically different with respect to their affinities for Gα* and their catalytic activity, respectively, and are independently activated by Gα*. Occupancy of the high-affinity Gα*-binding site induces very low cGMP hydrolytic activity, whereas occupancy of the low-affinity Gα*-binding site induces full cGMP hydrolytic activity. In model 2 (c), the two catalytic PDE6 subunits are functionally equal. High-affinity binding of the first Gα* to either of the two binding sites on the PDE6 induces a conformational change that confers very low activity to both catalytic sites. Full activation of both catalytic subunits requires low-affinity binding of a second copy of Gα*.