Figure 5.
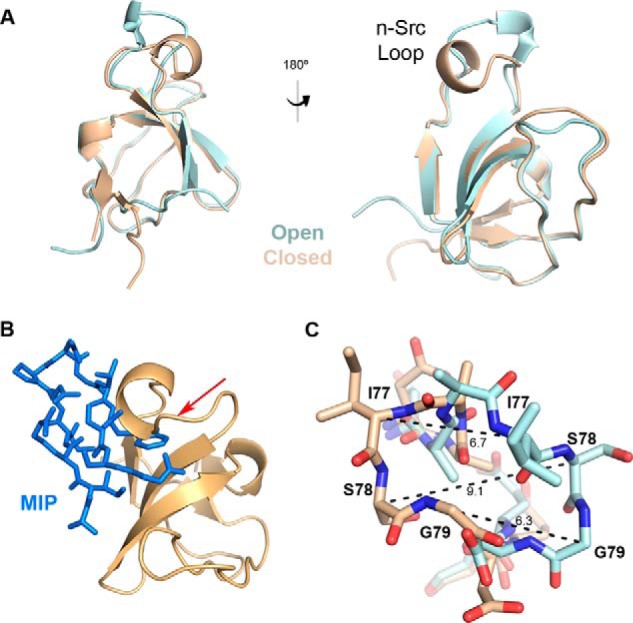
Conformational changes in the n-Src loop induced by MIP binding. A, crystal structure of apo MLK3 SH3(44–103) (tan) overlaid with MIP-bound MLK3 SH3(41–105) domain (cyan) shows a dramatic shift in the n-Src loop region, whereas the structure of the remainder of the domain is relatively unchanged. B, the n-Src loop of the unbound form of MLK3 SH3 must open up to allow MIP to bind the SH3 domain. Without this conformational change, MIP (blue) would not fit into the domain as evidenced by the clash indicated by the red arrow. C, a close-up of residues Ile-77, Ser-78, and Gly-79 in the n-Src loop in the bound (cyan) versus unbound (tan) structures. Measurements (Å) of the change of positions of the α-carbons in these residues demonstrate the spatial movement required to facilitate the interaction with MIP.
