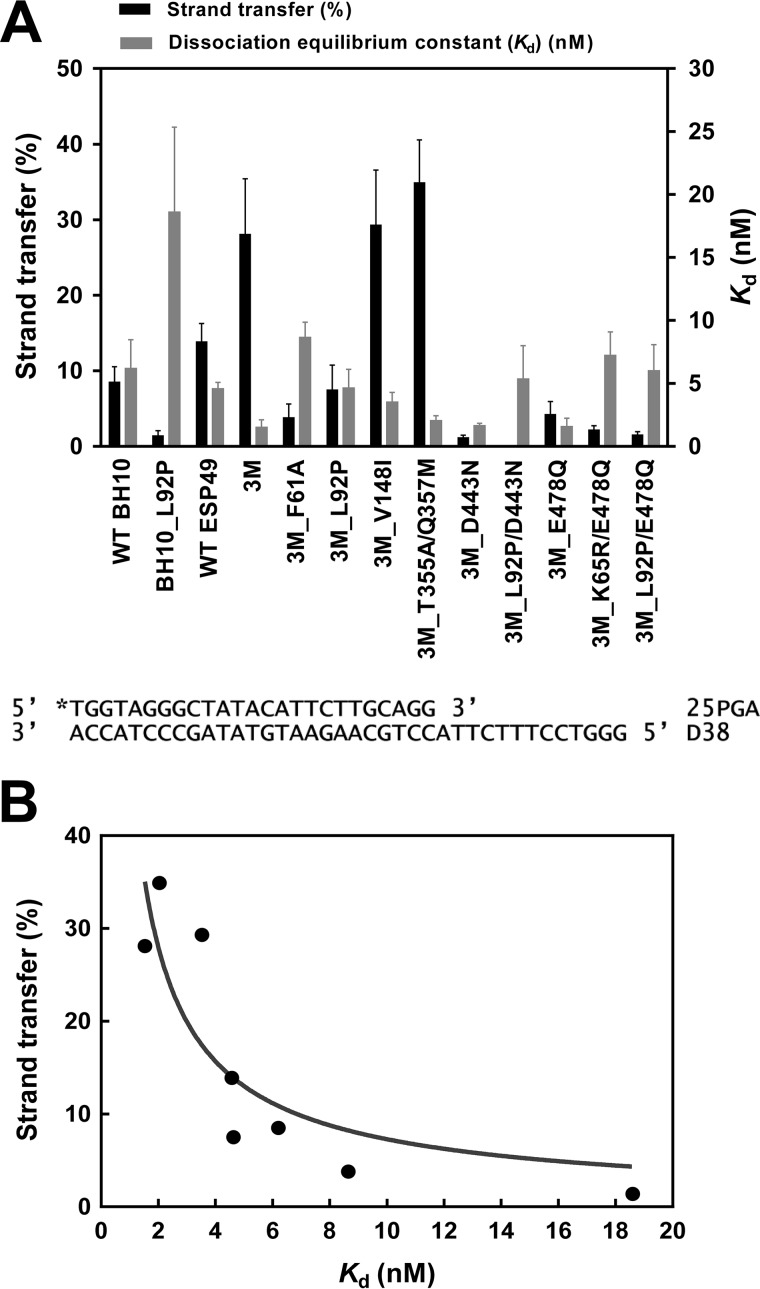
Figure 3.
Strand transfer efficiencies and dissociation equilibrium constants (Kd) of WT and mutant HIV-1 RTs. A, black bars represent strand transfer efficiencies (average ± S.D.) of all RTs, obtained after incubating the reaction during 60 min using DNA acceptor (35D). Gray bars indicate the mean Kd values ± S.D. for all RTs. Kd values (obtained with template-primer D38/25PGA) were calculated by fitting the data to the quadratic form of the binding equation as described under “Experimental procedures.” Represented values were obtained from at least three independent experiments. B, correlation between the strand transfer efficiencies and binding affinities of all tested enzymes. RTs containing RNase H–inactivating mutations (i.e. all those having D443N or E478Q) were devoid of strand transfer activity and therefore were excluded from the analysis. The correlation was found to be significant (Pearson r test, p < 0.05).