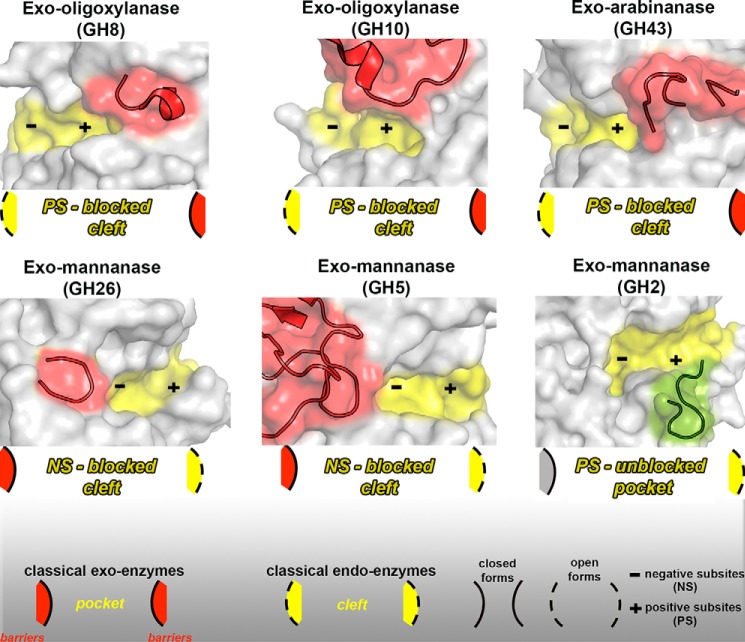
Figure 11.
Molecular mechanisms adopted by exo-enzymes derived from classical endo- or exo-GH members. The GH8 exo-oligoxylanase (PDB code 1WU4 (34)) presents an emergent loop (red cartoon) formed by the residues Ser317–Leu323 that blocks the positive-subsite region, resulting in a blocked cleft (represented in the scheme). A similar mechanism is adopted by the GH10 exo-oligoxylanase (PDB code 4PMV (21)) (residues Val267–Pro303) and by the GH43 exo-arabinanase (PDB code 4KCB (35)) (residues Arg203–Asn231). In the GH26 (PDB code 2VX5 (32)) and GH5 (PDB code 1UUQ (20)) exo-mannanases, a steric barrier imposed by the emergent loops (Arg201–Asn231 and Trp378–Phe412, respectively) at the negative-subsite regions confers the exo mode of action and also results in a blocked cleft. In the XacMan2A (PDB code 6BYC), a nonblocking loop (green cartoon) (residues Gly534–Thr544) at the positive-subsite region disrupts the classical pocket found in GH2 enzymes and gives rise to extra positive subsites. All emergent blocking loops are represented in red cartoons. Active-site cavities are highlighted in yellow. Negative (NS) and positive subsites (PS) are represented by a minus and plus sign, respectively.