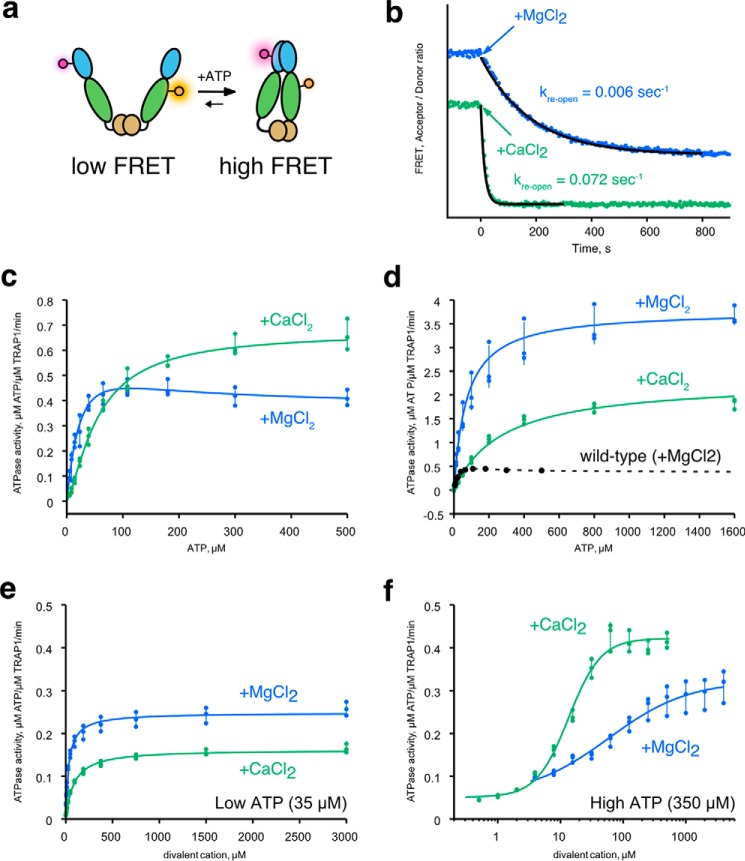
Figure 1.
Calcium supports ATP hydrolysis by TRAP1. a, cartoon diagram of the FRET assay with TRAP1 to monitor open (low FRET) and closed states (high FRET). Dye molecules (glowing circles) are covalently attached at the middle domain (green ellipse) and the NTD (blue ellipse). b, a representative FRET re-opening assay starting from the closed state (high FRET). Reaction was initiated by addition of either MgCl2 (blue) or CaCl2 (green) at t = 0. Time-dependent decrease of FRET signal indicate dimer opening. FRET is quantified as the ratio of acceptor/donor intensity. The kinetic trace has been vertically offset for clarity. Black lines are exponential fits to the re-opening kinetics with indicated rate constants, kre-open. Measurements were repeated at least three times. c, ATPase activity of hTRAP1 in presence of excess MgCl2 (blue) or CaCl2 (green) as a function of ATP concentration. d, ATPase activity of WT:D158N heterodimeric mixture in the presence of MgCl2 or CaCl2 as a function of ATP concentration. WT ATPase data with MgCl2 (black circles) and fit (dashed line) is shown for comparison. e, ATPase activity of hTRAP1 at low ATP concentration (35 μm) as a function of divalent cation concentrations (MgCl2 in blue; CaCl2 in green). f, ATPase activity of hTRAP1 at high ATP concentration (350 μm) as a function of divalent cation concentrations (MgCl2 in blue; CaCl2 in green). The error bars from c–f are standard deviations calculated from the data points shown as scatter plots.