Figure 4.
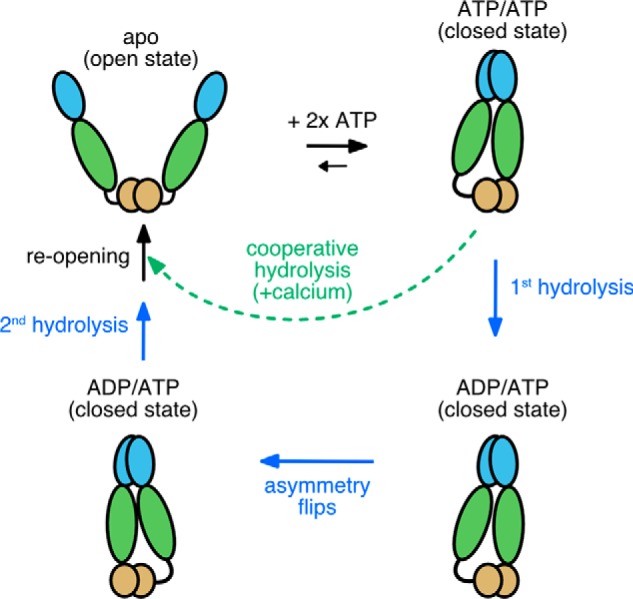
Model for TRAP1 ATPase cycle showing the normal sequential two-step ATP hydrolysis mechanism observed with magnesium (solid blue arrows and text) and an alternative path (dashed green arrow and text) when using calcium. The open dimer binds 2 ATPs, which in turn stabilizes an asymmetric closed state. Calcium binding at least partially bypasses the magnesium-supported pathway for stepwise hydrolysis, resulting in cooperative ATPase activity that consequently skips the conformational switch that happens after the first ATP hydrolysis.
