Abstract
Atezolizumab is a humanized anti-PD-L1 immune checkpoint antibody that is currently used in many kinds of advanced carcinoma including metastatic non-small cell lung cancer. The cutaneous side effect profile reported only 20$ of the patients which had only mild maculopapular rash that required no treatment. There is no case report of anti-PD-L1 antibody-induced Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) eruptions. To the best of our knowledge, there is no case report of atezolizumab-induced SJS or SJS/TEN induced by anti-PD-L1 immune checkpoint antibodies. We believe that our report will be useful to dermatologists who are consultants in the inpatient settings, as atezolizumab is an anti-neoplastic agent that has a potential to be used in multiple malignancies.
Keywords: Drug eruption, Drug hypersensitivity syndrome, Stevens-Johnson syndrome, Lung cancer, Lung cancer treatment, Cancer
Atezolizumab (MPDL3280A), a humanized anti-PD-L1 monoclonal immunoglobulin G1 antibody, is currently the only Food and Drug Administration-approved PD-L1 inhibitor in the United States for the treatment of patients with metastatic non-small cell lung cancer and advanced urothelial carcinoma whose disease progressed despite platinum-containing chemotherapy [1]. The cutaneous side effect profile for PD-L1 inhibitors has been found to be similar to PD-1 inhibitors. Skin toxicities mainly manifest in the form of maculopapular rash and pruritus but more characteristic dermatologic complications can also occur, including vitiligo, lichenoid dermatitis, exacerbated psoriasis, and mucosal involvement (e.g., lichenoid reaction, xerostomia) [2]. A recent study by McDermott et al. [3] evaluating the safety and toxicity of atezolizumab in 70 patients treated for renal cell carcinoma found that most patients experienced grade I–II of immune-related adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) grading scale, and the most common immune-related adverse event was a grade I rash (20$) defined as maculopapular eruptions with total body surface area involvement less than 10$ and without limitations of activities of daily living. Most patients required no treatment for their cutaneous side effect [3].
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but potentially fatal complications of anti-PD-1 therapy. To date, there are 2 cases of nivolumab-induced TEN [4, 5], 1 case of SJS after radiotherapy with anti-PD-1 therapy [6], and 2 cases of pembrolizumab-induced SJS [7]. To the best of our knowledge, there is no case report of anti-PD-L1 immune checkpoint antibody-induced SJS/TEN eruptions. We present a case of atezolizumab-induced SJS, which we believe is the first case report of SJS/TEN induced by anti-PD-L1 immune checkpoint antibodies in a patient with non-small cell lung carcinoma.
A 75-year-old male with stage IV non-small cell lung adenocarcinoma (T3N2M1) with bone and multiple lymph node metastases developed a non-pruritic rash over the trunk, which progressed to the upper extremities with oral erosions on day 2 of cycle 2 of atezolizumab treatment. The atezolizumab regimen was planned to be given for a total of 6 monthly cycles, each cycle lasting 3 days, and was given as an investigative treatment for his non-small cell lung cancer. Physical examination showed erythematous papules and plaques on the trunk and upper extremities with erosions on the lower lip. The skin lesions resolved after the completion of cycle 2; therefore, cycle 3 was planned to begin as scheduled. On day 1 of cycle 3, the patient developed bullous eruptions with positive Nikolsky's sign, oral mucositis and conjunctivitis (Fig. 1, 2, 3). The total body surface area of detachment was 5$. Skin biopsy from the lesion on the back showed superficial and deep perivascular infiltrates of lymphocytes and eosinophils, with unfortunately loss of epidermal sheet from the section (Fig. 4). The patient was then diagnosed with SJS. Laboratory tests including complete blood counts, BUN, creatinine, and liver function test were within normal limits. Atezolizumab was discontinued. Intravenous dexamethasone 5 mg was administered every 12 h, and chloramphenicol eye drops were initiated. He was discharged after 8 days of hospitalization with significant clinical improvement and was continued on oral prednisolone at 20 mg per day followed by a 1-week taper course. He achieved complete resolution after approximately 2 weeks from treatment initiation. In terms of his lung cancer, the patient had stable disease after 3 cycles of atezolizumab.
Fig. 1.
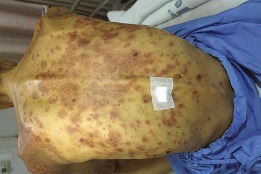
Multiple ill-defined non-blanchable papules and macules coalescing to form plaques with some central necrosis and flaccid bullae on the back.
Fig. 2.
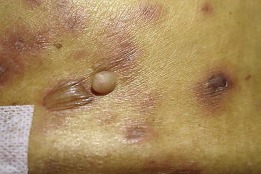
Flaccid bullae on the back showing positive Nikolsky's sign.
Fig. 3.
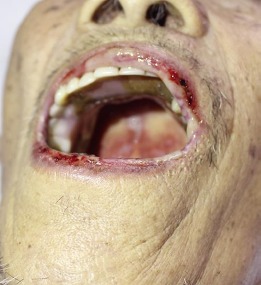
Multiple crusted erosions on the lips and shallow ulcers on the palate and buccal mucosa.
Fig. 4.
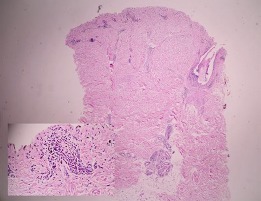
Histologic sections (40× and 200×) showing superficial and deep perivascular infiltrate of lymphocytes and eosinophils.
SJS/TEN results in massive keratinocyte apoptosis mediated by cytolytic molecules, including FasL, perforin/granzyme B, annexin A1, and granulysin. Histologically, SJS and TEN are characterized by full-thickness epidermal necrolysis due to extensive keratinocyte apoptosis associated with varying degrees of inflammation and epidermal infiltration by CD8+ lymphocytes.
The mechanism of SJS/TEN associated with anti-PD-1/PD-L1 immune checkpoint antibodies remains unclear. PD-1/PD-L interactions play a crucial role in T-cell homeostasis of the skin which prevents severe skin-directed inflammatory reactions. Thus, it is speculated that PD-1 antagonism results in the loss of T-cell homeostasis within the skin, thereby causing self-directed cytotoxic and inflammatory reactions [8]. This hypothesis was supported by Vivar et al. [5] and Goldinger et al. [9]. The former showed a significant increase in the expression of PD-L1 in lymphocytes and keratinocytes after the 3rd cycle of nivolumab (anti-PD-1 immune checkpoint antibodies) treatment as skin eruptions progressed from morbilliform eruptions to TEN. The latter demonstrated that immune checkpoint inhibitors can activate T cells and target keratinocytes leading to apoptosis which manifests clinically as TEN [9]. The mainstay of SJS/TEN treatment is supportive care, which includes identification and discontinuation of potential offending medications, appropriate wound care, maintenance of fluids and electrolytes homeostasis, temperature control, and consultation of appropriate specialties (ophthalmology and urology) for evaluation and support [10]. Adjuvant therapies are warranted in severe cases. Recent data from a systematic review and meta-analysis of systemic immunomodulating therapies (including glucocorticosteroids, IVIG, cyclosporine, plasmapheresis, thalidomide, cyclophosphamide, hemoperfusion, TNF inhibitors, and G-CSF) found that glucocorticosteroids and cyclosporine are the most promising systemic immunomodulating therapies for SJS/TEN [10]. In our case, the patient responded well to a systemic glucocorticosteroid beginning at an equivalent dose of prednisolone 1 mg/kg/day followed by a 2-week taper regimen. Complete resolution was achieved within approximately 2 weeks after treatment initiation.
We report the first known case of SJS resulting from anti-PD-L1 therapy. Early recognition and treatment is key to management in patients with similar skin eruptions. Further work is needed to elucidate the exact molecular pathways of anti-PD-L1 therapy in the pathogenesis of SJS/TEN in order to develop the optimal management of patients with anti-PD-L1-specific skin eruptions.
Statement of Ethics
According to the regulations of Mahidol University, this case report was exempted from undergoing review by the Institutional Review Board and the use of informed consent.
Disclosure Statement
No funding was utilized, and all authors have no financial relationships to report.
References
- 1.Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to antiprogrammed cell death 1 and anti-programmed cell death ligand 1 immunotherpay. JAMA Dermatol. 2016 Oct;152((10)):1128–36. doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016 Jul;28((4)):254–63. doi: 10.1097/CCO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 3.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an anti-programed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016 Mar;34((8)):833–42. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 4.Nayar N, Briscoe K, Fernandez Penas P. Toxic epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016 Apr;39((3)):149–52. doi: 10.1097/CJI.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 5.Vivar KL, Deschaine M, Messina J, Divine JM, Rabionet A, Patel N, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017 Apr;44((4)):381–4. doi: 10.1111/cup.12876. [DOI] [PubMed] [Google Scholar]
- 6.Liniker E, Menzies AM, Kong BY, Cooper A, Ramanujam S, Lo S, et al. Activity and safety of radiotherapy with anti-PD-1 drug therapy in patients with metastatic melanoma. OncoImmunology. 2016 Aug;5((9)):e1214788. doi: 10.1080/2162402X.2016.1214788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. 2017 Aug;81:237–9. doi: 10.1016/j.ejca.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Okiyama N, Katz SI. Programmed cell death 1 (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J Autoimmun. 2014 Sep;53:1–9. doi: 10.1016/j.jaut.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res. 2016 Aug;22((16)):4023–9. doi: 10.1158/1078-0432.CCR-15-2872. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017 Jun;153((6)):514–22. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]


