Figure 1.
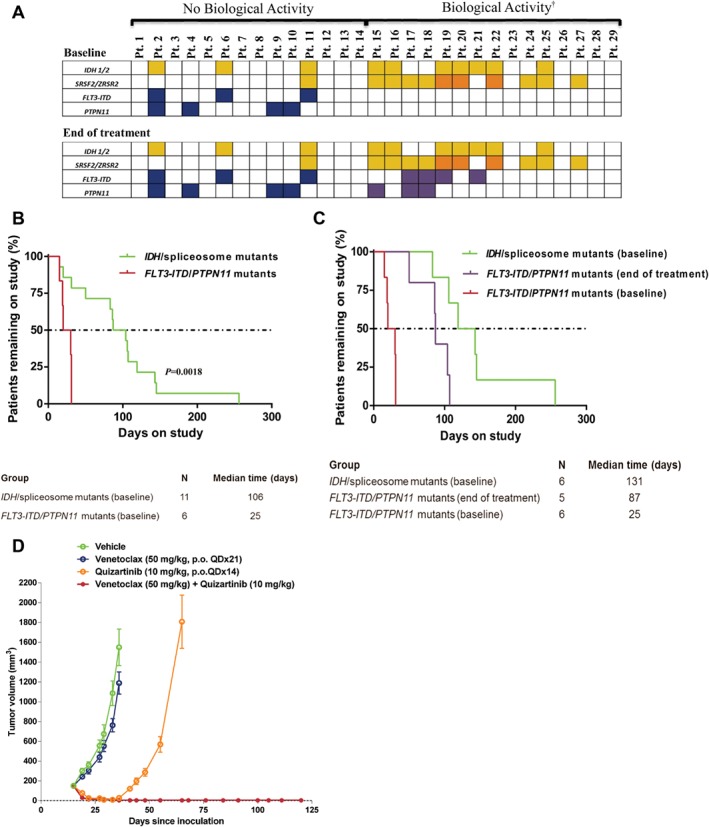
Mutations affecting response to venetoclax in AML. A, Mutations observed pre‐therapy and end of treatment with single agent venetoclax therapy. Yellow indicates IDH or SRSF2 mutations; orange indicates ZRSR2 mutations; blue indicates FLT3‐ITD or PTPN11 mutations; purple indicates newly detected FLT3‐ITD or PTPN11 mutations; blank cells indicate specific mutation was not detected, †Biological activity defined as any reduction in BM blast count while on venetoclax therapy; B, Time on study for patients with mutations associated with intrinsic sensitivity (pre‐therapy IDH/spliceosome mutants) and intrinsic resistance (pre‐therapy FLT3‐ITD/PTPN11 mutants) to venetoclax; C, Time on study for patients with mutations associated with intrinsic sensitivity, intrinsic resistance, and acquisition of mutations associated with acquired resistance (FLT3‐ITD/PTPN11 mutants) to venetoclax; D, Tumor growth inhibition by venetoclax plus quizartinib in mice xenografted with FLT3‐ITD+ MV‐4–11 cells
