Fig. 1:
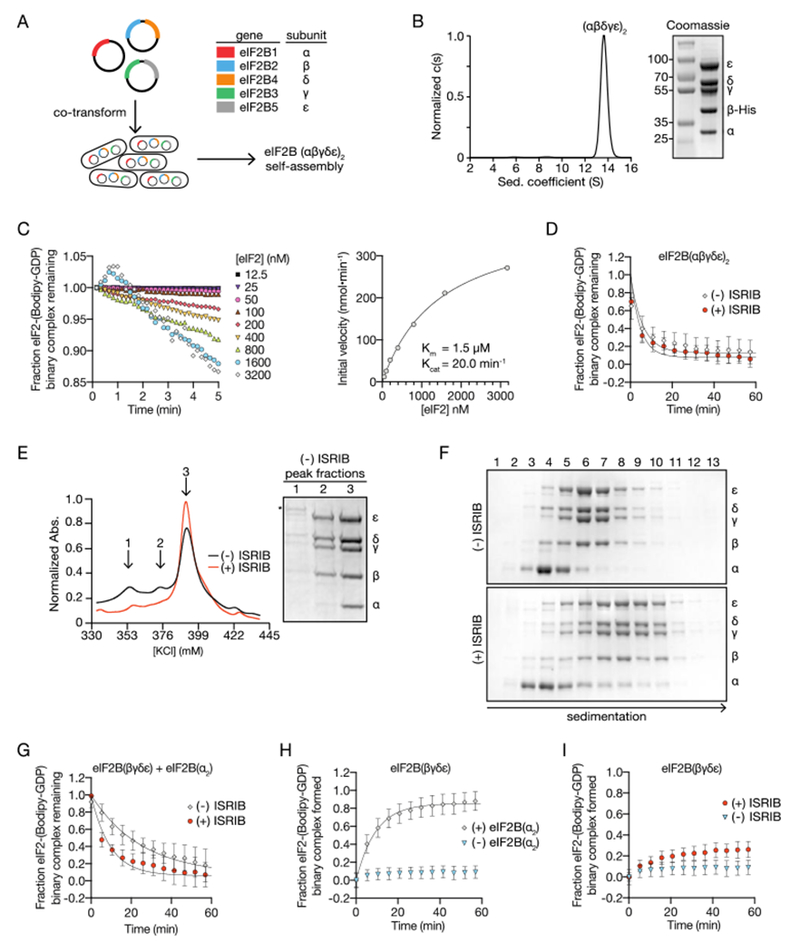
ISRIB stabilizes decameric eIF2B, accelerating GEF activity. (A) Schematic diagram for three plasmid expression of all five eIF2B genes in E. coli. (B) Characterization of eIF2B(αβγδε)2 by sedimentation velocity analytical ultracentrifugation and SDS-PAGE followed by Coomassie blue staining. (C) Initial rate of nucleotide exchange (right panel) plotted as a function of substrate concentration. Note that at high eIF2 concentration we reproducibly observed a transient increase in fluorescence that peaked at the 1 min time point (left panel). Such increase was reported previously (29) and remains unexplained. (D) GEF activity of eIF2B(αβγδε)2 as measured by unloading of fluorescent GDP from eIF2 in the presence and absence of ISRIB. (E) Absorbance 280 nm trace from an anion exchange column used in the purification of eIF2B in the presence (red) and absence (black) of ISRIB. Peak fractions from the (−) ISRIB purification were analyzed by SDS-PAGE and Coomassie-stained. eIF2B subunits are labeled (α-ε) and an asterisk denotes the presence of a contaminating protein that contributes to peak 1. (F) Stability of eIF2B(αβγδε)2 was assessed by sedimentation velocity on a 5-20% sucrose gradient in a 400 mM salt buffer. eIF2B(βγδε) and eIF2B(α2) were combined with and without 500 nM ISRIB. Fractions were analyzed by SDS-PAGE and Coomassie-stained. (G) GEF activity of eIF2B assembled from purified eIF2B(βγδε) and eIF2B(α2) in the presence and absence of ISRIB. (H) GEF activity of eIF2B(βγδε) in the presence and absence of eIF2B(α2). (I) GEF activity of eIF2B(βγδε) in the presence and absence of ISRIB.
