Fig. 5:
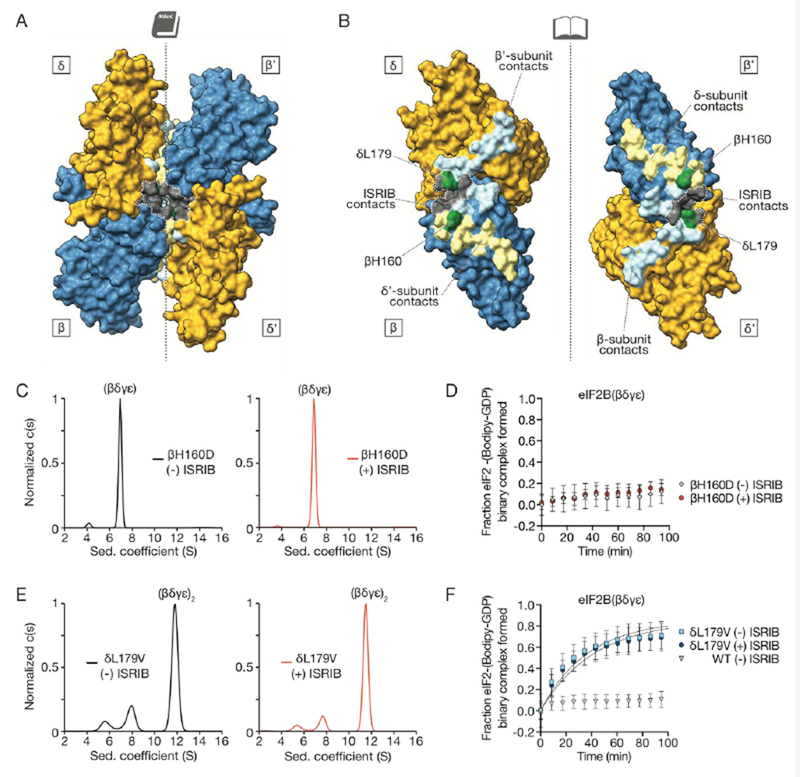
Loss- and gain-of-function dimerization mutants resist or bypass the effects of ISRIB. (A) Surface rendering of core eIF2Bβ (blue) and eIF2Bδ (gold) subunits with residues contacting ISRIB highlighted in gray and with dimer interface indicated by dashed line. Interface residues are highlighted in a lighter hue of the colors of the contacting subunits. (B) Open-book view of the dimer-dimer interface, such that each β and δ subunit is rotated by 90˚. βH160, in green, contacts both β’ and δ’; δL179, also in green, contacts both β’ and ISRIB. (C) Characterization of 1 μM eIF2B(βγδε) containing a βH160D mutation in the presence (right) and absence (left) of 1 μM ISRIB by analytical ultracentrifugation. (D) GEF activity of eIF2B(βγδε) containing a βH160D mutation in the presence and absence of ISRIB. (E) Characterization of 1 μM eIF2B(βγδε) containing a δL179V mutation in the presence (right) and absence (left) 1 μM ISRIB by analytical ultracentrifugation. (F) GEF activity of eIF2B(βγδε) containing a δL179V mutation in the presence and absence of ISRIB.
