Figure 3.
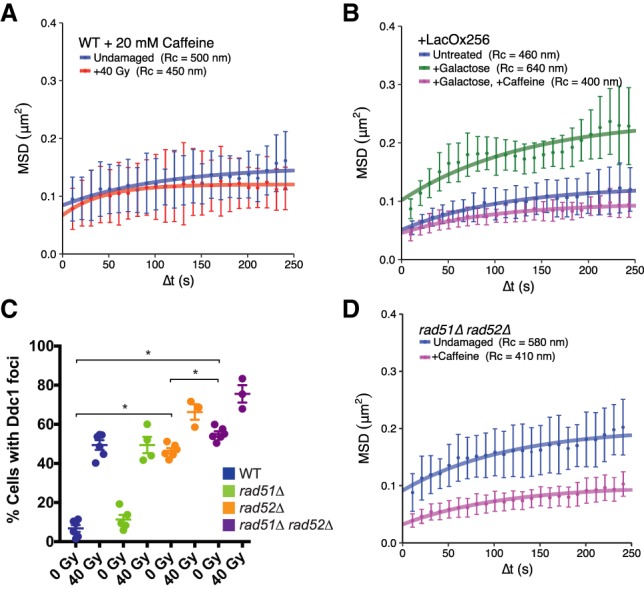
rad51Δ rad52Δ cells display elevated mobility as a result of an activated DNA damage checkpoint. (A) Both undamaged (blue) and irradiated (red) cells treated with 20 mM caffeine show similar confinement radii, demonstrating that a checkpoint response is required for global mobility. (B) Colocalization of Ddc1-LacI and Ddc2-LacI (expressed after their induction by galactose) leads to artificial checkpoint activation and global mobility in the absence of DNA damage (see the Materials and Methods; Supplemental Fig. S1A). Strains containing a lacO array for colocalization of these sensors (either uninduced [blue], induced [green], or induced in the presence of caffeine [magenta]) are shown. A control strain in which the lacO array has not been included does not undergo global mobility after galactose induction (Supplemental Fig. S1B). (C) Counts of Ddc1 foci in undamaged and damaged wild-type, rad51Δ, rad52Δ, and rad51Δ rad52Δ cells. Each point represents the percentage of cells with Ddc1 foci in a given experiment. Error bars are mean ± SEM. (*) P-value < 0.05, as calculated by unpaired t-test. (D) Undamaged rad51Δ rad52Δ cells treated with 20 mM caffeine (magenta) and untreated (blue) show that constitutive mobility in the double mutant is due to an activated checkpoint. Mobility experiments in A and B were performed in Rad52-CFP-tagged cells, while those in D were performed in Ddc1-CFP-tagged cells. Error bars of MSD plots represent the 95% CI.
