Abstract
A 72-year-old Japanese woman diagnosed with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis was admitted to our hospital with hearing loss, temporal pain, and sudden blindness. We finally diagnosed recurrent granulomatosis with polyangiitis and initiated methyl-prednisolone pulse therapy (1,000 mg) followed by prednisolone (30 mg/day) and rituximab (RTX). After the third RTX administration, she developed bloody stools along with acute thrombocytopenia and low complement levels. We diagnosed rituximab-induced acute thrombocytopenia (RIAT), and her platelet counts spontaneously recovered. This case suggests that after RTX therapy RIAT may sometimes cause severe thrombocytopenia, and that monitoring the complements may be useful for making an early diagnosis of RIAT.
Keywords: rituximab-induced acute thrombocytopenia, granulomatosis with polyangiitis
Introduction
Granulomatosis with polyangiitis (GPA) is a necrotizing vasculitis on small- to medium-sized vessels that often forms granulomases and systemic inflammatory disease that affects a wide range of organ systems. In GPA, immune deposits on the vessels are generally absent, and serum antineutrophil cytoplasmic antibody (ANCA) is frequently positive. The effectiveness of rituximab (RTX) against ANCA-associated vasculitis (AAV) has been clarified, and it is now widely used for the induction and maintenance of remission (1,2). However, the long-term safety of RTX as a treatment for AAV remains to be elucidated.
RTX is a chimeric murine/human anti-CD20 monoclonal antibody. RTX attacks CD20-positive B cells by several mechanisms, including complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) (3). The production of antibodies such as ANCA is thus suppressed by suppressing the differentiation of B cells into plasma cells. RTX is generally administered to patients as multiple infusions, and infusion-related side effects such as fever, chills, rash, pruritis, nausea, headache, hypotension, and bronchospasm are well known; they usually occur within a few hours of the initiation of the first infusion (4). As hematological abnormalities due to RTX, neutropenia, thrombocytopenia and anemia have been described, which generally occur during the first 10-14 days after the infusion (5). Grade 3/4 levels of neutropenia, thrombocytopenia, and anemia have been reported in only 4.2%, 1.7%, and 1.1% of patients, respectively (6).
Rituximab-induced acute thrombocytopenia (RIAT) that occurs within a few days of RTX administration has been reported in a minority of patients with B-cell non-Hodgkin's lymphomas (5,7-17), especially mantle cell lymphomas (7-15). Among the reports of autoimmune diseases, RIAT has been reported in a rituximab-treated patient with autoimmune hemolytic anemia (18), but our review of the relevant literature did not identify any AAV patients with RIAT. We herein report the case of a patient with GPA who developed RIAT after RTX treatment.
Case Report
In October 2015, a 72-year-old Japanese woman was diagnosed with AAV at another hospital based on the findings of right otitis media, interstitial pneumonia, and a high titer of myeloperoxidase (MPO)-ANCA (18.4 IU/mL). She was administered prednisolone (PSL) 60 mg/day, and she achieved clinical remission within a few weeks. Since she maintained remission, the dose of PSL was gradually tapered to 8 mg/day.
In January 2017, she presented with hearing loss in her right ear, right temporal pain, and sudden blindness in her right eye. She was referred to the ophthalmology department of our hospital, and hypertrophic pachymeningitis was revealed by contrast magnetic resonance imaging (MRI) in April 2017. She was then admitted to our department for further evaluation in May 2017. On admission, a physical examination revealed right vision deterioration (with no light perception) and a hearing impairment. Laboratory investigations showed the following results: white blood cell count (WBC) 10,800 /μL (Neutrophil 81.2%, Lymphocytes 12.7%), hemoglobin (Hb) 10.8 g/dL, platelet (PLT) 24.1×104/μL, C-reactive protein (CRP) 5.82 mg/dL, erythrocyte sedimentation rate (ESR) 80 mm/h, rheumatoid factor (RF) 57.5 IU/mL, and no abnormalities of urinalysis or renal dysfunction.
The following immunological and serological results were all negative: antinuclear antibody (ANA), proteinase-3 anti-neutrophil cytoplasmic autoantibodies (PR3-ANCAs), myeloperoxidase anti-neutrophil cytoplasmic autoantibodies (MPO-ANCAs), IgG4, and angiotensin converting enzyme (ACE). The results of a β-D-glucan assay and a T-SPOT.TB assay were all negative. A thoracico-abdominal computed tomography examination and a nerve conduction velocity test revealed no abnormalities.
Contrast MRI revealed a right intraorbital mass, swelling of right extraocular muscles with abnormal signals of the nasal cavity and maxillary sinus, and hypertrophic pachymeningitis from the frontal lobe to the temporal lobe on the same side. A cerebrospinal fluid test showed no elevation of the cell number or protein levels and no abnormality on bacteriological and cytological examinations, except for the interleukin (IL)-6 level, which reflects intracranial inflammation. Although the levels of MPO-ANCA and PR3-ANCA were not increased, we diagnosed recurrent GPA based on the patient's past medical history of confirmed AAV and the findings of recurrent otitis media, sinusitis, an intraorbital mass, and hypertrophic pachymeningitis.
Accordingly, we administered 1,000 mg of methyl-prednisolone pulse therapy followed by prednisolone 30 mg/day with tapering and the infusion of RTX (375 mg per m2 of body-surface area per week). After the first RTX administration, the patient developed an infusion reaction consisting of transient tachycardia and mild hypotension. We then adjusted the administration rate, and she was therefore able to continue receiving RTX infusions. No detectable infusion reaction was observed during the subsequent RTX administration.
Just before the third RTX infusion, the patient nearly achieved a completed remission of the hearing loss, temporal pain, and the level of CRP (reduced to 0.43 mg/dL), and there were no significant changes in laboratory values (WBC 4,900 /μL, Hb 10.4 g/dL, PLT 15.9×104/μL). However, 3 days after the third RTX infusion she suddenly developed bloody stools along with acute pancytopenia, and particularly thrombocytopenia (WBC 1,800 /μL, Hb 9.0 g/dL, PLT 0.7×104/μL). We administered a 20-unit platelet transfusion, and the bleeding stopped promptly. There was no platelet aggregation or hemolysis in the patient's peripheral blood. Although the CRP level increased transiently, there were no findings to suggest disseminated intravascular coagulation (DIC) or infection such as cytomegalovirus. A bone marrow aspiration smear also showed no abnormality in cell density of three systems, such as blood cells, red blood cells, and platelets, and also there was no increase in the dysplasia or blast cells. Since the patient's acute clinical course did not suggest drug-induced acute pancytopenia other than that by RTX, we thus diagnosed RIAT.
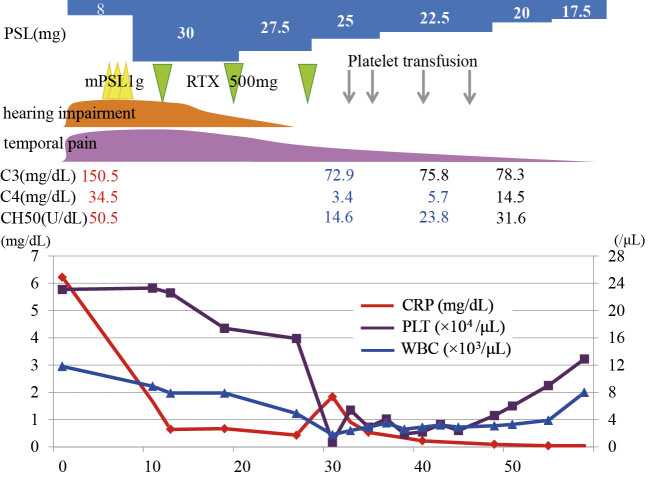
During the patient's clinical course after the RTX therapy, her complement levels declined as follows: C3, from 150.5 to 72.9 mg/dL (normal range 73-138); C4, from 34.5 to 3.4 mg/dL (normal range 11-31); and CH50, from 50.5 to 14.6 U/mL (normal range 30-46). She was administered a total of four platelet transfusions (total of 50 units), but 2 weeks later, her PLT counts spontaneously recovered to 4.6×104/μL, and at 3 weeks later to 9.0×104/μl in parallel with the complement levels (Figure). At the same time, the WBC and Hb counts also spontaneously recovered. We decided to discontinue any subsequent cycles of RTX to avoid the risk of bleeding, and we administered the combination of azathioprine 50 mg/day with tapering of the PSL. Her remission has now been maintained for over 6 months as of this writing, without any hematological abnormalities.
Figure.
The clinical course of the patient, a 72-year-old Japanese woman. The CRP, platelet, and WBC values and the treatment interventions during the hospital course are shown. mPSL: methyl-prednisolone, RTX: rituximab
Discussion
We treated a patient with recurrent GPA who developed RIAT after a third RTX administration. RTX has been widely used for CD20-positive B-cell lymphoproliferative diseases. Since the efficacy or RTX for patients with AAV has also been clarified, it was approved in 2013 in Japan for treating microscopic polyangiitis (MPA) and GPA. In clinical trials (RAVE, RITXVAS) comparing RTX and cyclophosphamide (CY) in patients with AAV, there were no significant differences in the remission induction rate or overall safety between RTX and CY (1,19). Regarding hematologic toxicity, grade 2 or higher leukopenia was observed more frequently in the CY group (RTX group 3%, CY group 10%), whereas grade 3 or higher thrombocytopenia was observed more frequently in the RTX group (RTX group 3%, CY group 1%) (1).
Accordingly, in the treatment of patients with AAV, clinicians should pay more attention to the presence of remarkable thrombocytopenia. In addition, the incidence of RTX-induced thrombocytopenia has been considered to be higher than that actually observed in clinical trials. It has also been reported that a low platelet count, a high platelet distribution width (PDW) before RTX administration, and the presence of neoplastic disease are risk factors for RTX-induced thrombocytopenia (20).
A previous report summarizing RTX-induced thrombocytopenia (RIT) showed that most of the RIT cases occurred within 1 month, especially within 10 days, after the last administration of RTX (20). Among the RIT cases, several cases with RIAT, which developed within a few days after RTX administration, have been reported (5,7-18), and are considered to be particularly important clinically. According to these reports, most of the patients with RIAT typically reached a nadir within 1 day and then resolved spontaneously within a few days (13). In our patient's case, it took 3 days for her to reach a nadir and approx. 2 weeks to resolve spontaneously. Among the previous reports of cases of acute cytopenias after the administration of RTX, thrombocytopenia was the most frequent adverse event, followed by leukocytopenia, anemia, and pancytopenia (13).
As an adverse event associated with RTX administration, infusion reactions are well known, as they are related to cytokine release syndrome (CRS). The severity of an infusion reaction is correlated with increased levels of inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-6, IL-8, and interferon-gamma (IFN-γ) (21). Although we did not measure the serum cytokines in our patient's case, a prior case report about RIAT also showed elevated TNF-α levels in the serum, and it was noted that 82% of the 12 reported RIAT cases were preceded by the clinical presentation of CRS, thereby suggesting an association between RIAT and CRS (13). In our case, infusion reaction was not observed after the last administration of RTX, but the CRP level increased transiently at the onset of RIAT, thus suggesting that CRS was involved in the development of RAIT.
RTX has the ability to bind to C1q, activating a complement cascade which may lead to an accumulation of complement activation products and cytokines (22). In turn, these events play central roles in infusion reactions and CRS (23,24). Since platelets do not express CD20 receptor, there is no association between RTX therapy and the direct platelet destruction or the induction of antiplatelet autoantibodies (13). Complement activation could cause acute thrombocytopenia through a direct degradation of platelets (25) or a downstream effect and the accumulation of some cytokines such as TNF-α (26). In our patient's case, a positive correlation between platelets and complement values was observed, suggesting that complement activation is involved in the pathogenesis of RIAT. It is unclear to what degree an immunological abnormality of this patient may have played a role in the present disease causing the onset of RIAT, but a clarification of this contribution could help elucidate the association between the pathogenesis of GPA and other types of vasculitis (such as MPA) and the onset of RAIT.
In conclusion, we treated a patient with significant acute thrombocytopenia that occurred during RTX treatment for GPA. The frequency of RTX treatment by rheumatologists is expected to increase in the future. However, the current clinical experience with RTX treatment for AAV is still insufficient to make any definitive conclusions, and thus it is necessary to recognize the potential adverse events and to manage them appropriately. For high-risk patients who may develop CRS post-RTX administration, careful monitoring including that of complements in the serum is recommended for the early diagnosis of RIAT.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 221-232, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 371: 1771-1780, 2014. [DOI] [PubMed] [Google Scholar]
- 3. Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol 30: 3-8, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 14: E10-E21, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Ureshino H, Nishioka A, Kojima K, et al. Rituximab-induced acute thrombocytopenia in high tumor burden follicular lymphoma. Intern Med 55: 2061-2064, 2016. [DOI] [PubMed] [Google Scholar]
- 6. McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16: 2825-2833, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Shah C, Grethlein SJ. Case report of rituximab-induced thrombocytopenia. Am J Hematol 75: 263, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Yi JH, Kim SJ, Ahn HK, et al. Rituximab-induced acute thrombocytopenia: a case report and review of the literature. Med Oncol 26: 45-48, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Rosado M, Chao H, Rose M. Severe acute thrombocytopenia following rituximab therapy. Leuk Lymphoma 48: 2239-2240, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Dhand S, Bahrain H. Rituximab-induced severe acute thrombocytopenia: a case report and review of literature. Cancer Invest 26: 913-915, 2008. [DOI] [PubMed] [Google Scholar]
- 11. El-Osta H, Nair B. Rituximab-induced acute thrombocytopenia: an underappreciated entity. Leuk Lymphoma 54: 2736-2737, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Sadashiv SK, Rao R, Fazal S, Lister J. Rituximab-induced acute severe thrombocytopenia: a case series in patients with mantle cell lymphoma. Clin Lymphoma Myeloma Leuk 13: 602-605, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Ram R, Bonstein L, Gafter-Gvili A, Ben-Bassat I, Shpilberg O, Raanani P. Rituximab-associated acute thrombocytopenia: an under-diagnosed phenomenon. Am J Hematol 84: 247-250, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Parajuli R, Hire E, Shah BK. Rituximab-induced acute severe thrombocytopenia. Br J Haematol 149: 804, 2010. [DOI] [PubMed] [Google Scholar]
- 15. Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica 90(Suppl): ECR23, 2005. [PubMed] [Google Scholar]
- 16. Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90: 2188-2195, 1997. [PubMed] [Google Scholar]
- 17. Rigamonti C, Volta C, Colombi S, et al. Severe thrombocytopenia and clinical bleeding associated with rituximab infusion in a lymphoma patient with massive splenomegaly without leukemic invasion. Leukemia 15: 186-187, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Larrar S, Guitton C, Willems M, Bader-Meunier B. Severe hematological side effects following Rituximab therapy in children. Haematologica 91: ECR36, 2006. [PubMed] [Google Scholar]
- 19. Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 363: 211-220, 2010. [DOI] [PubMed] [Google Scholar]
- 20. Giezen TJ, Mantel-Teeuwisse AK, ten Berg MJ, et al. Rituximab-induced thrombocytopenia: a cohort study. Eur J Haematol 89: 256-266, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 19: 2153-2164, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83: 435-445, 1994. [PubMed] [Google Scholar]
- 23. van der Kolk LE, de Haas M, Grillo-Lopez AJ, Baars JW, van Oers MH. Analysis of CD20-dependent cellular cytotoxicity by G-CSF-stimulated neutrophils. Leukemia 16: 693-699, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Gutierrez A, Rodriguez J, Martinez J, et al. Pathogenic study of anti-CD20 infusion-related severe refractory shock in diffuse large B-cell lymphoma. Leuk Lymphoma 47: 111-115, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Shibazaki M, Kawabata Y, Yokochi T, Nishida A, Takada H, Endo Y. Complement-dependent accumulation and degradation of platelets in the lung and liver induced by injection of lipopolysaccharides. Infect Immun 67: 5186-5191, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michelmann I, Bockmann D, Nurnberger W, Eckhof-Donovan S, Burdach S, Gobel U. Thrombocytopenia and complement activation under recombinant TNF alpha/IFN gamma therapy in man. Ann Hematol 74: 179-184, 1997. [DOI] [PubMed] [Google Scholar]