FIGURE 3.
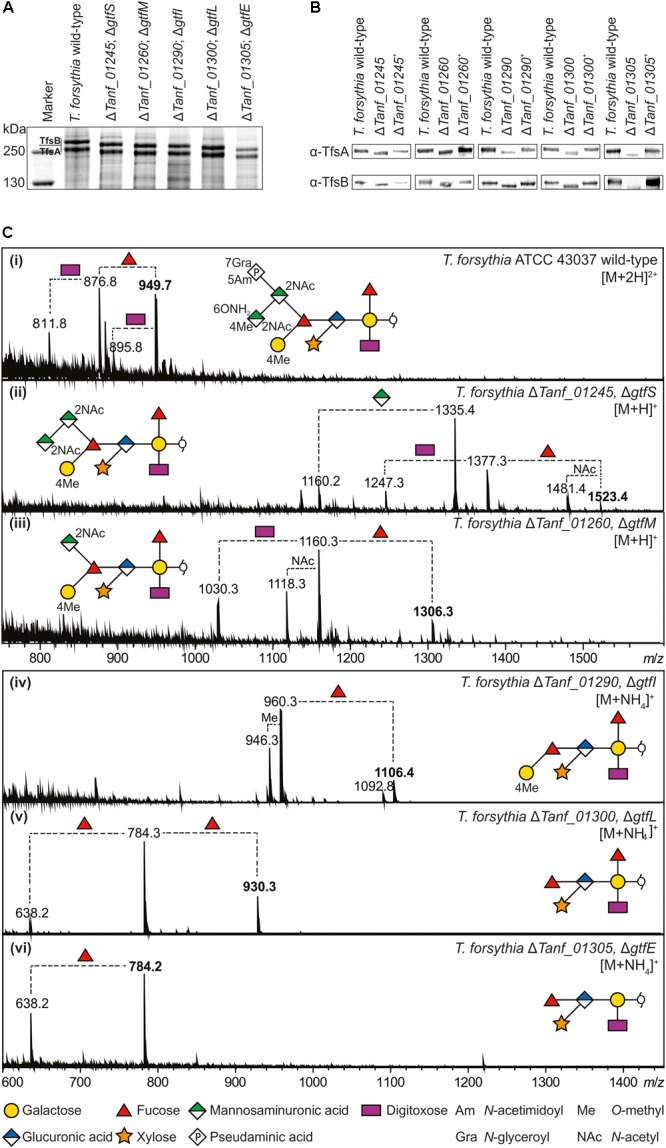
(A) Coomassie Brilliant Blue staining of crude cell extracts from T. forsythia ATCC 43037 wild-type and glycosyltransferase-deficient mutants after separation on a 7.5% SDS-PA gel. The S-layer glycoproteins (labeled TfsA and TfsB) are indicated and the downshifts resulting from glycan truncation can be seen in the mutants. S-layer glycoprotein bands were further processed for MS analyses. PageRuler Plus Prestained Protein Ladder (Thermo Fisher Scientific) was used as a protein molecular weight marker. (B) Western-blots probed with α-TfsA and α-TfsB antiserum for confirmation of the identity of S-layer glycoproteins. Glycoproteins from all glycosyltransferase-deficient mutants (ΔgtfSMILE) experienced a downshift resulting from glycan truncation, whereas the reconstituted strains (denoted with +) regained wild-type migration, indicating the presence of the complete mature glycan, proving successful recombination. (C, i–vi) ESI-MS sum spectra of β-eliminated TfsB O-glycans from T. forsythia wild-type and mutants. The glycan structures of the signals corresponding to the largest mass (bold m/z values) are shown in SNFG representations (Varki et al., 2015). O-Glycan signals detected for the respective mutants were assigned based on the m/z mass differences corresponding to the loss of individual sugar units and/or modifications.
