Abstract
Background
It is a matter of debate whether, and if so, to what extent, cancer screening programs reduce all-cause mortality. Against this backdrop, we analyzed potential effects of several cancer screening approaches on all-cause mortality in two representative Western European populations.
Methods
We used mortality data from the UK (England & Wales) and Germany from 2015 and published figures from screening studies on relative reduction in mortality for screened cancers to calculate the expected decline in all-cause mortality in these countries. We determined the required sample size for demonstrating a 3% reduction in all-cause mortality with a narrow (95%) confidence interval in a hypothetical screening trial.
Results
A relative 20% reduction in breast cancer mortality can be accompanied by a maximum 1.7–1.8% reduction in all-cause mortality in England & Wales and Germany, respectively. Expected declines are smaller for sigmoidoscopy screening (1.0–1.2%), prostate-specific antigen (PSA) screening (0.4–0.6%), and skin cancer screening (0.2%). To obtain a 95% confidence interval of +/–1% for demonstrating a 3% decline in all-cause mortality, a study size of 596 200 persons is required.
Conclusion
Because the proportion of cancer deaths in all deaths in Western Europe is relatively low, cancer screening procedures can reduce all-cause mortality by only 1–3%. However, this reduction is relevant to public health.
There are three death categories for the calculation of mortality rates in screening studies: a) cancer-related death in a person suffering from the cancer of interest, b) death in a person suffering from the cancer of interest which is not attributed to the cancer of interest, and c) death of any cause in persons not suffering from the cancer of interest. Many screening studies report cancer-specific mortality rates (disease-specific mortality rates), which only takes category a) into account. Some screening studies also report all-cause mortality rates, taking into account deaths of all categories a)–c).
Imagine that in one study arm of a screening study a total of 10 000 person-years were spent on follow-up. 50 participants died of the cancer of interest. Another 30 participants, who also fell ill with the cancer of interest, died of another cause. Another 20 participants who did not fall ill with the cancer of interest during their lifetime died from another cause. The cancer-specific mortality rate is therefore calculated as 50 per 10 000 person-years, while the all-cause mortality rate is calculated as 100 per 10 000 person years.
The evaluation of the mortality in screening trials is dominated by the study of the cancer-specific mortality rate among screened and unscreened populations. However, several huge randomized screening trials also report all-cause mortality rates. There has been some debate about the choice of mortality data for the evaluation of screening trials. For example, Penston advocates the use of the all-cause mortality rate because information on the underlying cause of death is frequently unreliable and disease-specific mortality rate ignores that screening may cause death due to the detected cancer (1). Furthermore, there may be uncertainties in the assignment of disease-specific causes of death, since side effects or complications in the diagnosis and therapy of cancer are not necessarily assigned to the underlying disease, i.e. the cancer.
Opponents of the use of the all-cause mortality rate state that even common cancers account for only a small proportion of the total number of deaths and therefore screening trials would require sample sizes too large to be feasible. However, the expected decline in all-cause mortality after the introduction of cancer screening in populations like Western Europe have not been estimated (2).
Another debate concerns the question, what constitutes convincing evidence of a beneficial effect on the all-cause mortality rate in screening studies? Although nearly all screening studies are statistically underpowered to be able to show a small effect on all-cause mortality as statistically significant, several authors use statistical significance to judge whether a screening study shows an effect on all-cause mortality (3). Swartz speaks of an “inconsistency” between the effect on all-cause mortality and cancer-specific mortality without explaining when it exists (4).
We could not find any publication that quantifies the expected decline of the all-cause mortality rate if an efficacious screening for a specific cancer is introduced. Knowledge about the expected decline of the all-cause mortality rate helps to interpret results from statistically underpowered screening studies. For example, the European Randomized Study of Screening for Prostate Cancer (ERSPC) revealed that the prostate cancer-specific mortality rates among men aged 55–69 years over a period of 11 years decreases by 21% if prostate-specific antigen (PSA) screening is conducted every four years (5). This study showed that the all-cause mortality rates were very similar (screening: 18.2 per 1000 person-years; no screening: 18.5 per 1000 person-years; mortality rate ratio 0.99, 95% CI: [0.97; 1.01]). Based on these results, Schröder et al. stated, “In our study, there was no effect on all-cause mortality.” (5) Are Schröder et al. right?
The aim of this paper is to present the potential effect of cancer screening on all-cause mortality in Western Europe. We chose two representative countries for which recent mortality data were available. We hereby assess the role of the magnitude of the cancer mortality rate without screening and the relative reduction in the cancer mortality rate due to screening.
Material and methods
We extracted the most recent available mortality data (counts and population size) from Germany (2015) as provided by the Federal Statistical Office (www.gbe-bund.de, accessed January 24, 2018) and from the UK (England & Wales) (2015) provided by the Office for National Statistics (https://www.ons.gov.uk, accessed February 11, 2018) for screening-detectable cancers including colorectal cancer (International Classification of Diseases, 10th edition, ICD-10: C18–C21), skin melanoma (C43), breast cancer (C50), prostate cancer (C61), and for ischemic heart disease (I20–I25) (6).
We estimated age-standardized mortality rates for all-cause mortality and for screening-detectable cancers by use of the European Standard Population (7). We compared the sex- and age-specific mortality rates of these cancers graphically. We used estimates of the relative rate reduction (RRR) of cancer mortality for screenings that have been studied by large randomized controlled trials including PSA screening (age 55–69 years) (5), mammography screening (age 50–69 years) (8), and flexible sigmoidoscopy screening (age 55–64 years) (9).
We used a RRR of 21% for PSA screening (5), 20% for mammography screening (8), 27% for flexible sigmoidoscopy (9), and 50% for skin melanoma (10) respectively. For skin cancer, we only focused on skin melanoma deaths as non-melanoma skin cancer mortality rates are very low. We used the RRR to estimate cancer-specific mortality rates, assuming a scenario where screening is applied to 100% of the eligible population, and thereafter calculated the expected all-cause mortality rate, accounting for the expected reduction in cancer-specific mortality due to screening. This calculation assumes that the all-cause mortality rate is directly influenced only by the change in the cancer-specific mortality rate. Indirect effects such as, for example, suicide after a diagnosis of cancer may decrease the effect.
We thereafter calculated the mortality rate ratio (rate in presence of screening/rate without screening) for all-cause mortality.
In addition, we investigated the hypothetical effect of screening in ischemic heart disease (ICD-10: I20–I25), whose disease-specific mortality rate (and therefore its proportion of all-cause mortality) in the 45–69 year-old age group is considerably higher than that of cancer. In a sensitivity analysis, we assumed that the screening effect would also be noticeable in the 5-year age group above the approved screening age (e.g. in mammography screening the group of 70–74 year-olds) if we used the same RRR for this group.
In order to analyze the dependence between the relative rate reduction of a screening program for a specific cancer and the cancer mortality rate of this cancer without screening, which would exist for a reduction in all-cause mortality by 1%, 2%, or 3%, respectively, we derived a mathematical formula.
To calculate the required sample size for estimating a relative risk of 0.98 or 0.97 of all-cause mortality in a hypothetical screening trial with a narrow two-sided 95% confidence interval of +/–0.01, we used the confidence interval method by Katz et al. (11). We assumed equal group sizes for screened and unscreened participants of a hypothetical randomized controlled screening trial.
Results
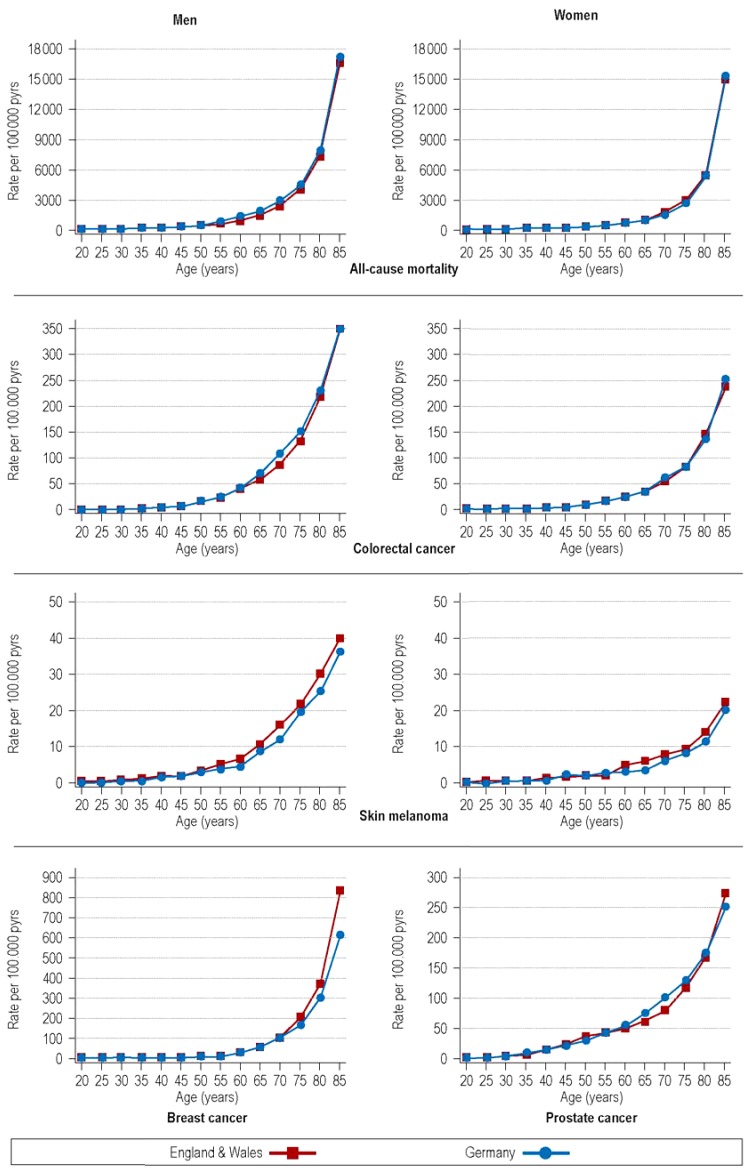
Although there were slight differences in age-standardized all-cause mortality rates between the UK (England & Wales) and Germany (men: 617 versus 687 per 100 000 person-years; women: 453 versus 448 per 100 000 person-years, respectively), the age-specific mortality rates of the cancers studied here were very similar (eTable 1, eFigure).
eTable 1. Total and cancer-specific mortality rates (per 100 000 person-years) in the UK (England & Wales) and Germany in 2015.
|
Crude rates per 100 000 person-years |
Age-standardized rates per 100 000 person-years |
|||||||||
| Germany | England & Wales | Germany | England & Wales | |||||||
| Deaths | Rate | SE | Deaths | Rate | SE | Deaths | SE | Rate | SE | |
| Men | ||||||||||
| Total | 449 512 | 1119 | 1.7 | 257 207 | 901 | 1.8 | 687 | 1.1 | 617 | 1.3 |
| Colorectal cancer | 13 649 | 34.0 | 0.3 | 7773 | 27.2 | 0.3 | 20.6 | 0.2 | 19.0 | 0.2 |
| Melanoma | 1767 | 4.4 | 0.1 | 1323 | 4.6 | 0.1 | 2.8 | 0.1 | 3.4 | 0.1 |
| Prostate cancer | 13 900 | 34.6 | 0.3 | 10 575 | 37.0 | 0.4 | 19.4 | 0.2 | 23.2 | 0.2 |
| Women | ||||||||||
| Total | 475 688 | 1146 | 1.7 | 272 448 | 929 | 1.8 | 448 | 0.7 | 453 | 1.0 |
| Colorectal cancer | 11 769 | 28.4 | 0.3 | 6640 | 22.6 | 0.3 | 12.4 | 0.1 | 12.6 | 0.2 |
| Melanoma | 1287 | 3.1 | 0.1 | 884 | 3.0 | 0.1 | 1.6 | 0.1 | 1.9 | 0.1 |
| Breast cancer | 18 136 | 43.7 | 0.3 | 10 169 | 34.7 | 0.3 | 23.0 | 0.2 | 22.3 | 0.2 |
Age-standardized rates are standardized by the European Standard Population; SE, standard error of the rate
eFigure.
Age-specific mortality rates of screening-detectable cancers in Germany and the UK (England & Wales), 2015
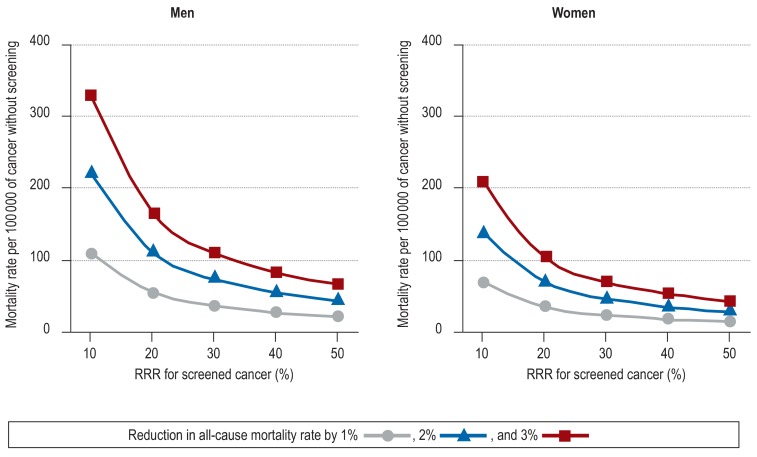
A cancer screening procedure among people aged 50–74 years with a relative rate reduction (RRR) in cancer-specific mortality of e.g. 20% that would also be associated with a reduction in all-cause mortality by 1%, 2%, or 3% among men would require a mortality rate of that cancer without screening of 55, 110, or 165 per 100 000 person-years, respectively, in the UK (England & Wales) and Germany (women: 35, 69, or 104 per 100 000 person-years, respectively) (figure).
Figure.
Relation between the relative rate reduction of a screening program for a cancer and the mortality rate of a cancer without screening to reach a relative 1%, 2%, or 3% reduction in all-cause mortality among people aged 50–74 years in the UK (England & Wales) and Germany. The all-cause mortality rate among men aged 50–74 years in the UK (England & Wales) and Germany is approximately 1100 per 100 000 person-years; all-cause mortality among women aged 50–74 years in the UK (England & Wales) and Germany is about 690 per 100 000 person-years; RRR: relative rate reduction in cancer-specific mortality due to screening; reduction in the all-cause mortality rate of 1% (▲), 2% (■), and 3% ()
The Table shows the expected decline in all-cause mortality among screening-eligible populations with 100% participation in a cancer-specific screening programme for the UK (England & Wales) and Germany for colorectal cancer, breast cancer, prostate cancer, and skin melanoma. The proportion of cancer-specific mortality among all-cause deaths is 8.7% and 9.2% for women aged 50–69 years in the UK (England & Wales) and Germany, respectively.
Table. Total and cancer-specific mortality rates (per 100 000 person-years) and estimated effect of screening in the eligible population assuming 100% participation in the UK (England & Wales) and Germany 2015.
| Crude mortality rates | Expected all-cause mortality rate with screening | ||||
| Total | Cancer-specific rate | Rate | Change (%) | Rate ratio | |
| Sigmoidoscopy screening (men and women, 55 – 64 years) and colorectal cancer death (RR = 0.73) | |||||
| UK (England & Wales) | 621 | 26.7 | 614 | –1.2 | 0.99 |
| Germany | 743 | 27.6 | 736 | –1.0 | 0.99 |
| Mammography screening (women, 50 – 69 years) and breast cancer death (RR = 0.80) | |||||
| UK (England & Wales) | 528 | 46.2 | 519 | −1.7 | 0.98 |
| Germany | 517 | 47.5 | 508 | −1.8 | 0.98 |
| PSA screening (men, 55 – 69 years) and prostate cancer death (RR = 0.79) | |||||
| UK (England & Wales) | 976 | 28.7 | 970 | -0.6 | 0.99 |
| Germany | 1200 | 24.7 | 1.195 | -0.4 | 1.00 |
| Skin cancer screening (men and women, 35 – 85+ years) and skin melanoma death (RR = 0.50) | |||||
| UK (England & Wales) | 1599 | 6.6 | 1.595 | −0.2 | 1.00 |
| Germany | 1766 | 5.8 | 1.763 | −0.2 | 1.00 |
| Hypothetical screening for ischemic heart disease (men and women, 45 – 69 years) and death due to ischemic heart disease (RR = 0.75) | |||||
| UK (England & Wales) | 556 | 70.8 | 538 | −3.2 | 0.97 |
| Germany | 614 | 63.4 | 598 | −2.6 | 0.97 |
Change (%) compares the all-cause mortality rate with screening with the rate without screening; the rate ratio expresses the ratio of the all-cause mortality rate with screening with that without screening; PSA, prostate-specific antigen, RR, relative risk
Despite these high proportions, a RRR in breast cancer mortality within that age group would produce a relative decline in the all-cause mortality rate of only 1.7% and 1.8% in the UK (England & Wales) and Germany, respectively. Relative declines in the all-cause mortality rate would be smaller for sigmoidoscopy screening (1.0–1.2%), PSA screening (0.4–0.6%), and skin cancer screening (0.2%). A hypothetical screening for ischemic heart disease among people aged 45–69 years with an accompanying 25% RRR would result in a decline in the all-cause mortality rate of almost 3.2% and 2.6% in the UK (England & Wales) and Germany (Table, eTable 2).
eTable 2. All-cause mortality rates and cancer-specific mortality rates (per 100 000 person-years) and estimated effect of screening in the eligible population assuming 100% participation in the UK (England & Wales) and Germany in 2015*.
| Number of deaths and percentages | Crude mortality rates |
Expected all-cause mortality rate with screening |
|||||||
| Population | All deaths |
Cancer specific deaths |
% | Total |
Cancer specific rate |
Rate | Change (%) | Rate ratio | |
| Sigmoidoscopy screening (men and women, 55 – 64 years) and colorectal cancer death (RR = 0.73) | |||||||||
| England & Wales | |||||||||
| 55–59 | 3 475 497 | 16 693 | 735 | 4.4 | 480 | 21.1 | 475 | –1.2 | 0.99 |
| 60–64 | 3 089 643 | 24 093 | 1015 | 4.2 | 780 | 32.9 | 771 | –1.1 | 0.99 |
| 55–64 | 6 565 140 | 40 786 | 1750 | 4.3 | 621 | 26,7 | 614 | –1.2 | 0.99 |
| Germany | |||||||||
| 55–59 | 5 945 895 | 34 940 | 1257 | 3.6 | 588 | 21.1 | 582 | –1.0 | 0.99 |
| 60–64 | 5 177 524 | 47 758 | 1815 | 3.8 | 922 | 35.1 | 913 | –1.0 | 0.99 |
| 55–64 | 11 123 419 | 82 698 | 3072 | 3.7 | 743 | 27.6 | 736 | –1.0 | 0.99 |
| Mammography screening (women, 50 – 69 years) and breast cancer death (RR = 0.80) | |||||||||
| England & Wales | |||||||||
| 50–54 | 2 039 645 | 5094 | 728 | 14.3 | 250 | 35.7 | 243 | –2.9 | 0.97 |
| 55–59 | 1 757 445 | 6714 | 736 | 11.0 | 382 | 41.9 | 374 | –2.2 | 0.98 |
| 60–64 | 1 576 695 | 9675 | 775 | 8.0 | 614 | 49.2 | 604 | –1.6 | 0.98 |
| 65–69 | 1 652 275 | 15 632 | 1006 | 6.4 | 946 | 60.9 | 934 | –1.3 | 0.99 |
| 50–69 | 7 026 060 | 37 115 | 3245 | 8.7 | 528 | 46.2 | 519 | –1.7 | 0.98 |
| Germany | |||||||||
| 50–54 | 3 422 898 | 8524 | 1042 | 12.2 | 249 | 30.4 | 243 | –2.4 | 0.98 |
| 55–59 | 2 981 779 | 12 006 | 1243 | 10.4 | 403 | 41.7 | 394 | –2.1 | 0.98 |
| 60–64 | 2 661 828 | 16 773 | 1420 | 8.5 | 630 | 53.3 | 619 | –1.7 | 0.98 |
| 65–69 | 2 167 248 | 20 788 | 1627 | 7.8 | 959 | 75.1 | 944 | –1.6 | 0.98 |
| 50–69 | 11 233 753 | 58 091 | 5332 | 9.2 | 517 | 47.5 | 508 | –1.8 | 0.98 |
| PSA screening (men, 55 – 69 years) and prostate cancer death (RR = 0.79) | |||||||||
| England & Wales | |||||||||
| 55–59 | 1 718 052 | 9979 | 161 | 1.6 | 581 | 9.4 | 579 | –0.3 | 1.00 |
| 60–64 | 1 512 948 | 14 418 | 372 | 2.6 | 953 | 24.6 | 948 | –0.5 | 0.99 |
| 65–69 | 1 560 546 | 22 346 | 842 | 3.8 | 1432 | 54.0 | 1421 | –0.8 | 0.99 |
| 55–69 | 4 791 546 | 46 743 | 1375 | 2.9 | 976 | 28.7 | 970 | –0.6 | 0.99 |
| Germany | |||||||||
| 55–59 | 2 964 116 | 22 934 | 260 | 1.1 | 774 | 8.8 | 772 | –0.2 | 1.00 |
| 60–64 | 2 515 696 | 30 985 | 625 | 2.0 | 1232 | 24.8 | 1226 | –0.4 | 1.00 |
| 65–69 | 2 003 151 | 35 868 | 964 | 2.7 | 1791 | 48.1 | 1780 | –0.6 | 0.99 |
| 55–69 | 7 482 963 | 89 787 | 1849 | 2.1 | 1200 | 24.7 | 1195 | –0.4 | 1.00 |
| Skin cancer screening (men and women, 35 – 85+ years) and skin melanoma death (RR = 0.50) | |||||||||
| England & Wales | |||||||||
| 35–39 | 3 641 593 | 3326 | 38 | 1.1 | 91 | 1.0 | 91 | –0.6 | 0.99 |
| 40–44 | 3 826 336 | 5243 | 63 | 1.2 | 137 | 1.6 | 136 | –0.6 | 0.99 |
| 45–49 | 4 103 459 | 8405 | 77 | 0.9 | 205 | 1.9 | 204 | –0.5 | 1.00 |
| 50–54 | 4 030 657 | 12 458 | 116 | 0.9 | 309 | 2.9 | 308 | –0.5 | 1.00 |
| 55–59 | 3 475 497 | 16 693 | 133 | 0.8 | 480 | 3.8 | 478 | –0.4 | 1.00 |
| 60–64 | 3 089 643 | 24 093 | 183 | 0.8 | 780 | 5.9 | 777 | –0.4 | 1.00 |
| 65–69 | 3 212 821 | 37 978 | 272 | 0.7 | 1182 | 8.5 | 1178 | –0.4 | 1.00 |
| 70–74 | 2 419 031 | 47 385 | 288 | 0.6 | 1959 | 11.9 | 1953 | –0.3 | 1.00 |
| 75–79 | 1 919 669 | 64 792 | 292 | 0.5 | 3375 | 15.2 | 3368 | –0.2 | 1.00 |
| 80–84 | 1 410 234 | 87 108 | 297 | 0.3 | 6177 | 21.1 | 6166 | –0.2 | 1.00 |
| 85+ | 1 374 590 | 212 146 | 395 | 0.2 | 15 433 | 28.7 | 15 419 | –0.1 | 1.00 |
| 35–85+ | 32 503 530 | 519 627 | 2154 | 0.4 | 1599 | 6.6 | 1595 | –0.2 | 1.00 |
| Germany | |||||||||
| 35–39 | 4 855 188 | 3613 | 39 | 1.1 | 74 | 0.8 | 74 | –0.5 | 0.99 |
| 40–44 | 5 087 191 | 6083 | 60 | 1.0 | 120 | 1.2 | 119 | –0.5 | 1.00 |
| 45–49 | 6 623 073 | 13 409 | 153 | 1.1 | 202 | 2.3 | 201 | –0.6 | 0.99 |
| 50–54 | 6 905 717 | 24 213 | 183 | 0.8 | 351 | 2.6 | 349 | –0.4 | 1.00 |
| 55–59 | 5 945 895 | 34 940 | 207 | 0.6 | 588 | 3.5 | 586 | –0.3 | 1.00 |
| 60–64 | 5 177 524 | 47 758 | 201 | 0.4 | 922 | 3.9 | 920 | –0.2 | 1.00 |
| 65–69 | 4 170 399 | 56 656 | 261 | 0.5 | 1359 | 6.3 | 1355 | –0.2 | 1.00 |
| 70–74 | 4 197 712 | 87 833 | 383 | 0.4 | 2092 | 9.1 | 2088 | –0.2 | 1.00 |
| 75–79 | 4 189 609 | 139 965 | 561 | 0.4 | 3341 | 13.4 | 3334 | –0.2 | 1.00 |
| 80–84 | 2 460 564 | 156 003 | 424 | 0.3 | 6340 | 17.2 | 6332 | –0.1 | 1.00 |
| 85+ | 2 176 225 | 343 908 | 552 | 0.2 | 15 803 | 25.4 | 15 790 | –0.1 | 1.00 |
| 35–85+ | 51 789 097 | 914 381 | 3024 | 0.3 | 1766 | 5.8 | 1763 | –0.2 | 1.00 |
| Hypothetical screening for ischemic heart disease (men and women, 45–69 years) and death due to ischemic heart disease (RR = 0.75) | |||||||||
| England & Wales | |||||||||
| 45 | 4 103 459 | 8405 | 896 | 10.7 | 205 | 21.8 | 199 | –2.7 | 0.97 |
| 50 | 4 030 657 | 12 458 | 1579 | 12.7 | 309 | 39.2 | 299 | –3.2 | 0.97 |
| 55 | 3 475 497 | 16 693 | 2186 | 13.1 | 480 | 62.9 | 465 | –3.3 | 0.97 |
| 60 | 3 089 643 | 24 093 | 3208 | 13.3 | 780 | 103.8 | 754 | –3.3 | 0.97 |
| 65 | 3 212 821 | 37 978 | 4818 | 12.7 | 1182 | 150.0 | 1145 | –3.2 | 0.97 |
| 45–69 | 17 912 077 | 99 627 | 12 687 | 12.7 | 556 | 70.8 | 538 | –3.2 | 0.97 |
| Germany | |||||||||
| 45 | 6 623 073 | 13 409 | 1079 | 8.0 | 202 | 16.3 | 198 | –2.0 | 0.98 |
| 50 | 6 905 717 | 24 213 | 2192 | 9.1 | 351 | 31.7 | 343 | –2.3 | 0.98 |
| 55 | 5 945 895 | 34 940 | 3502 | 10.0 | 588 | 58.9 | 573 | –2.5 | 0.97 |
| 60 | 5 177 524 | 47 758 | 5111 | 10.7 | 922 | 98.7 | 898 | –2.7 | 0.97 |
| 65 | 4 170 399 | 56 656 | 6394 | 11.3 | 1359 | 153.3 | 1320 | –2.8 | 0.97 |
| 45–69 | 28 822 608 | 176 976 | 18 278 | 10.3 | 614 | 63.4 | 598 | –2.6 | 0.97 |
* Cancer-specific deaths: sigmoidoscopy—colorectal cancer deaths; mammography screening—breast cancer deaths; PSA screening—prostate cancer deaths; skin cancer screening—skin melanoma deaths; change (%) compares the all-cause mortality rate without screening with that rate with screening; the rate ratio [RR] expresses the ratio of the all-cause mortality rate with screening with that rate without screening
PSA, prostate-specific antigen
For all the diseases discussed here, there were hardly any changes after accounting for a potential carry-over effect of the mortality reduction to a higher age group for whom the respective screening is not provided. The maximum change in the percentage reduction in all-cause mortality due to a carry-over effect was 0.2% (Germany: prostate cancer, UK (England & Wales): breast cancer) (data not shown).
For a relative risk of 0.97, that is a relative risk reduction of 3%, related to all-cause mortality, the required study size of a screening trial with a two-sided 95% confidence interval of +/–0.01 is 596 200. For a relative risk of 0.98, the corresponding sample size is 602 346.
Discussion
We have shown that effective early detection of cancer in the age groups eligible for screening can hardly have an effect on all-cause mortality in two representative Western European populations in the 2010s. Therefore, statements on mammography screening such as “the all-cause mortality rate in the screening group is the same as that in the unscreened group”, “mammography does not save lives” (12), or “PSA screening increases harms without changing overall mortality” are incorrect as such differences can be expected to be small (2% or less) for two reasons:
The percentage of cancer deaths among all deaths is low, i.e. all-cause mortality is dominated by causes of death that are unrelated to the screened cancer.
Reduction in cancer mortality with screening is too low to substantially affect all-cause mortality.
The ERSPC study revealed that the prostate cancer–specific mortality rate among men aged 55–69 years over a period of 11 years decreased by 21% with PSA screening every four years (5). The all-cause mortality rates were, on the other hand, very similar (screening: 18.2 per 1000 person-years; no screening: 18.5 per 1000 person-years; mortality rate ratio 0.99, 95% CI: [0.97–1.01]). Based on these results, Schröder et al. concluded that “PSA-based screening reduces prostate cancer mortality but does not affect all-cause mortality.” Our analysis revealed that the estimated 1% decline in all-cause mortality found in the ERSPC study corresponds to a decline that can be expected based on the current all-cause mortality and prostate cancer-specific mortality rates in European populations such as the UK (England & Wales) and Germany in 2015. As only 2.9% (UK [England & Wales]) and 2.1% (Germany) of all deaths among men aged 55–69 years are due to prostate cancer, efficient prostate cancer screening can hardly influence the all-cause mortality rate.
Given an all-cause mortality rate of 621 and 743 per 100 000 person-years for men and women aged 55–64 years and a colorectal cancer mortality rate of 26.7 and 27.6 per 100 000 person-years within that age group in the UK (England & Wales) and Germany respectively, sigmoidoscopy screening even among 100% of eligible people cannot produce a decline in all-cause mortality by more than 1.0–1.2%. Skin cancer screening will hardly ever result in any appreciable decline in all-cause mortality as the percentage of deaths due to skin melanoma is simply too low (0.3–0.4% of all deaths among people aged 35 years or more) even in the presence of a large RRR of 50% as postulated by the SCREEN project (10).
To observe a decline in all-cause mortality among men aged 50–74 years by 3% for example, the cancer-specific mortality rate before screening for the cancer in question has to be about 110 per 100 000 person-years given a RRR of 30% (women aged 50–74 years: 69 per 100 000 person-years). However, none of the four cancers presented here has a mortality rate without screening that is in this order in the age groups eligible for screening. For ischemic heart disease, mortality rates among people aged 45–69 years are high and a hypothetical 25% RRR would result in a 3% decline in all-cause mortality in the UK (England & Wales) and Germany.
Our sample size calculations show that the study size of screening trials needs to be extremely large (half a million) in order to be able to demonstrate a reduction in all-cause mortality of 2–3% with a narrow (95%) confidence interval. As all published screening trials have sample sizes far below half a million, one cannot expect narrow confidence intervals for the RRR of all-cause mortality in screening trials. Consequently, one cannot expect ‘statistically significant’ declines in all-cause mortality. From a public health perspective, a 2% reduction in overall mortality is a relevant effect. If 100% of 50–69 year old women in Germany participated in mammography screening, the overall mortality rate would decrease from 517 per 100 000 person-years to 508 per 100 000 person-years (–1.8%) in that age group. Therefore, 9 per 100 000 deaths per year (1.8%) would be avoided in these women.
In conclusion, because the proportion of cancer deaths in all deaths in Western Europe is relatively low, cancer screening procedures can reduce all-cause mortality by only 1–3%. However, this reduction is of public health importance and clinically relevant. Furthermore, screening procedures can have a beneficial effect on non-lethal endpoints (aggressiveness of treatment, costs, etc.).
Statistical terms.
-
Age-specific mortality rate
Mortality rate (deaths per 100 000 person-years) within specific age groups
Age-standardized mortality rate Mortality rate (deaths per 100 000 person-years) age-standardized to the European Standard Population
Cancer-specific mortality rate Mortality rate (deaths per 100 000 person years), counting only deaths with cancer-related underlying disease of interest
-
Mortality rate ratio
The ratio of two mortality rates
-
RR
Relative risk; the risk of death of one group divided by the risk of death of another group
-
RRR
Relative rate reduction; the percentage reduction of the mortality rate due to screening
-
All-cause mortality rate
The all-cause mortality rate (deaths per 100 000 person years), includes all causes of death irrespective of whether someone was screened and irrespective of whether cancer was diagnosed during their lifetime.
Key messages.
In the current debate about the usefulness of cancer screening procedures, an effect on all-cause mortality is increasingly being considered a prerequisite for implementation.
An actual or presumed absence of effect on all-cause mortality is often mistakenly used as an argument against screening.
Even for effective cancer screening procedures, the expected reduction in all-cause mortality is only a few percent.
In addition to the fact that a reduction in all-cause mortality even by a few percent is relevant to public health, screening procedures can have a beneficial effect on non-lethal endpoints (aggressiveness of treatment, costs, etc.).
Footnotes
Conflict of statement
The authors declare that no conflict of interest exists.
References
- 1.Penston J. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ. 2011;343 doi: 10.1136/bmj.d6395. d6395. [DOI] [PubMed] [Google Scholar]
- 2.Steele RJ, Brewster DH. Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? No. BMJ. 2011;343 doi: 10.1136/bmj.d6397. d6397. [DOI] [PubMed] [Google Scholar]
- 3.Saquib N, Saquib J, Ioannidis JP. Does screening for disease save lives in asymptomatic adults? Systematic review of meta-analyses and randomized trials. Int J Epidemiol. 2015;44:264–277. doi: 10.1093/ije/dyu140. [DOI] [PubMed] [Google Scholar]
- 4.Swartz AW. Re: Why cancer screening has never been shown to “save lives“—and what we can do about it. BMJ. 2016;352 doi: 10.1136/bmj.h6080. h6080. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ., et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) WHO. 10. Geneva: 1992. International statistical classification of diseases and related health problems. [Google Scholar]
- 7.Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2:269–279. doi: 10.1002/ijc.2910020310. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) www.who.int/cancer/publications/mammography_screening/en (last accessed on 12 January 2018) Geneva WHO: 2014. WHO position paper on mammography screening. WHO library cataloguing-in-publication data; pp. 1–78. [Google Scholar]
- 9.Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US preventive services task force. JAMA. 2016;315:2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201–211. doi: 10.1016/j.jaad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Katz D, Baptista J, Azen SP, Pike MC. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978;34:469–474. [Google Scholar]
- 12.Grill M, Hackenbroch V. Unsinn in bester Qualität Der Spiegel. www.spiegel.de/spiegel/print/d-128239351.html (last accessed on 16 May 2018) 2014:100–104. [Google Scholar]