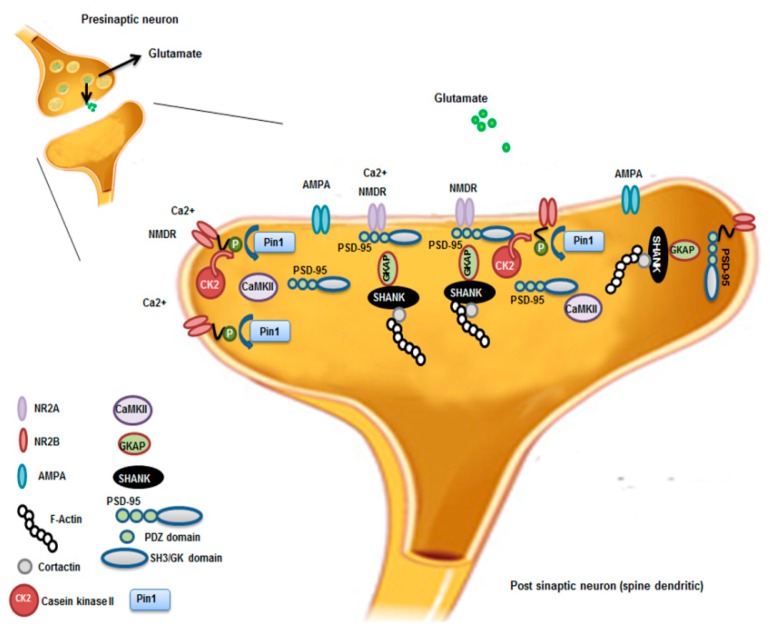
Figure 2.
Pin1 regulation of NMDAR complex and synaptic plasticity. Synaptic activation of NMDAR stimulates Ca2+/Calmodulin-dependent protein Kinase II (CaMKII) and casein kinase II (CK2) activity. CK2 phosphorylates the PDZ (postsynaptic density-95(PSD-95)/Discs large/zona occludens-1) ligand of NR2B. Pin1 binds and stabilizes probably the conformational change of NR2B phosphorylated disrupting the interaction between NMDAR on the cell surface and the PDZ domains of PSD-95. This leads to destabilization and internalization of surface NMDAR that influences, in turn, synaptic transmission and spine morphology. NR2A; NR2B; AMPA; F-Actin; Contarctin; CaMKII, Ca2+-Calmodulin dependent protein Kinase II; CK2, casein Kinase 2; PSD-95 presents three PDZ domains, SH3 (Sarc homology 3 domain) and GK (guanylate kinase-like domain) domains; Pin1; GKAP, Guanylate kinase associate protein; Shank, shank protein.