Table 1.
Structure of ring-substituted (2E)-N-aryl-3-phenylprop-2-enamides 1–16, experimentally determined values of lipophilicity log k, calculated values of log P/Clog P, and electronic Hammett’s σ parameters.
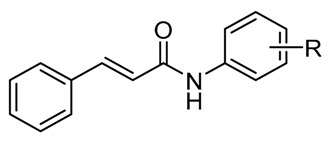
| Comp. | R | log k | Clog P a | log P b | σAr b |
|---|---|---|---|---|---|
| 1 | H | 0.1146 | 3.6640 | 3.18 | 0.60 |
| 2 | 3-CH3 | 0.2729 | 4.1630 | 3.40 | 0.48 |
| 3 | 4-CH3 | 0.2640 | 4.1630 | 3.40 | 0.46 |
| 4 | 2-F | 0.1330 | 3.4646 | 3.17 | 1.02 |
| 5 | 3-F | 0.2327 | 4.0646 | 3.32 | 0.82 |
| 6 | 3-CF3 | 0.4859 | 4.9978 | 4.26 | 0.89 |
| 7 | 2,5-CH3 | 0.2691 | 4.0120 | 3.57 | 0.59 |
| 8 | 2,5-Cl | 0.5799 | 4.5878 | 4.65 | 1.22 |
| 9 | 2,6-Cl | 0.0632 | 3.7378 | 4.56 | 1.33 |
| 10 | 3,4-Cl | 0.6821 | 5.3178 | 4.70 | 1.19 |
| 11 | 3,5-Cl | 0.8155 | 5.4378 | 4.79 | 1.11 |
| 12 | 2,6-Br | 0.0992 | 3.9778 | 4.80 | 1.33 |
| 13 | 3,5-CF3 | 0.9814 | 6.0386 | 5.68 | 1.05 |
| 14 | 2-F-5-Br | 0.4875 | 4.4178 | 4.07 | 1.28 |
| 15 | 2-Br-5-F | 0.4588 | 4.1378 | 4.12 | 1.19 |
| 16 | 2-Cl-5-CF3 | 0.6178 | 4.9509 | 4.88 | 1.19 |
a calculated using ChemBioDraw Ultra 13.0; bcalculated using ACD/Percepta ver. 2012.
