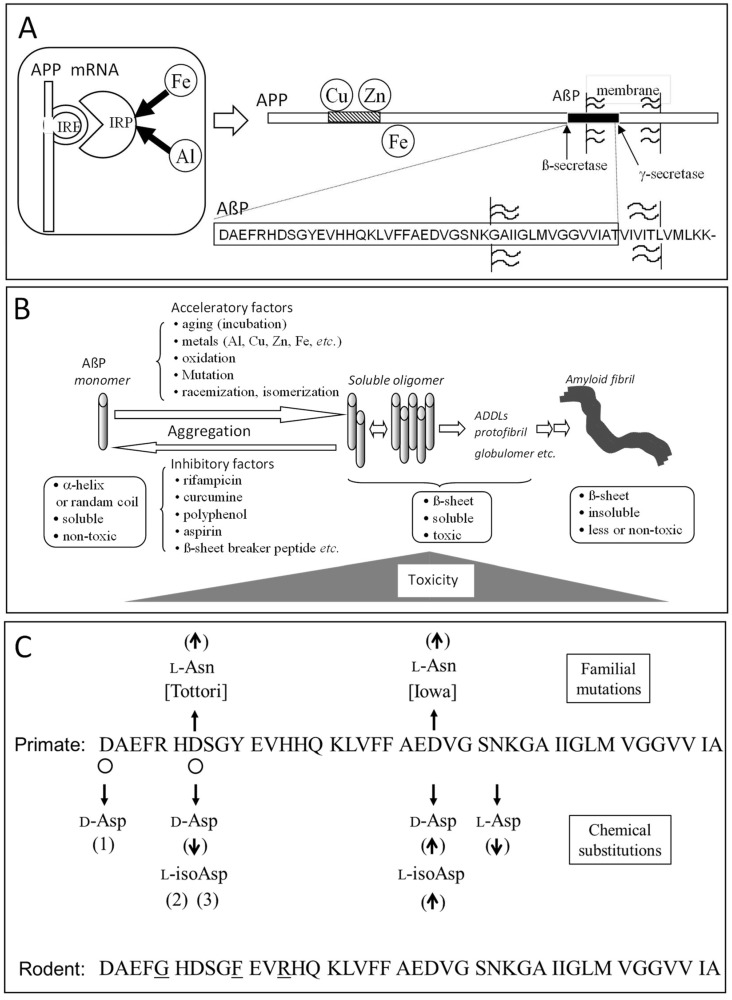
Figure 3.
Alzheimer’s disease and factors affecting AβP aggregation. (A) Structure of APP and AβP. AβP is secreted by cleavage from its precursor protein, APP by transmembrane cleavage. The mRNA of APP possesses IRE domain, and Fe regulates its expression. (B) AβP aggregation. AβP self-aggregates and forms several types of oligomers (including SDS-soluble oligomers, ADDLS, globulomers, or protofibrils) and finally forms insoluble aggregates termed amyloid fibrils. Oligomeric soluble AβPs are toxic, although the monomeric and fibril AβPs are rather nontoxic. The aggregation process is influenced by the acceleratory factors or the inhibitory factors. (C) Summary of Asp isomerization in AβP. The Asp isomerization positions found in an AD brain are indicated by open circles. The relationship between chemical substitution and fibril formation is also shown. (↑) acceleration or increase of fibril formation, (↓) suppression or decrease, (1) suppression of acceleration effect by Asp23 substitution [41], (2) unchanged in vitro assay, (3) triggered the dense-core congophilic amyloid plaque formation in APP transgenic mice. The comparison between the sequence of primate (human or monkey) AβP1–42 and rodent (rat or mouse) AβP1–42 is also depicted and the different amino acids are indicated by underline.