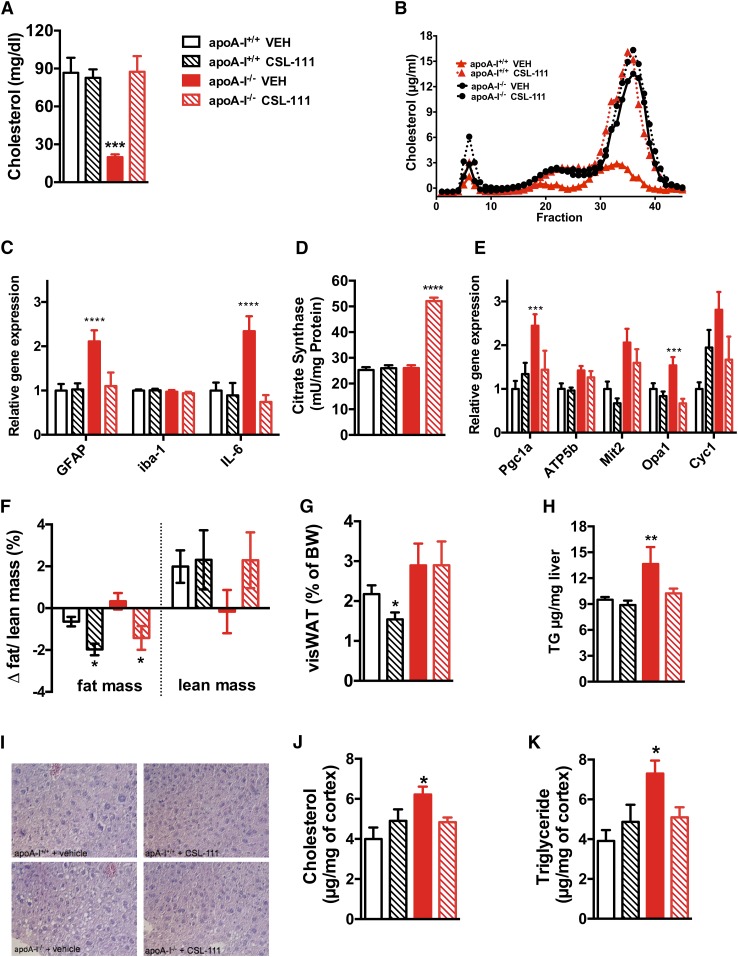
Fig. 5.
CSL-111 treatment normalizes hypothalamic inflammation and mitochondrial function markers in chow- fed apoA-I−/− mice. Plasma total cholesterol levels (A) and lipoprotein fractions (B) of apoA-I+/+ and apoA-I−/− mice after daily treatment with CSL-111 at 150 mg/kg for 7 days (n = 6 mice per group, age 7 months). CSL-111 normalizes hypothalamic inflammation (C) and reverses mitochondrial changes by enhancing citrate synthase activity (D) and normalizing mitochondrial function markers (E) in apoA-I−/− mice. Body composition analysis revealed reduced fat mass in apoA-I+/+ (white hatched bars) and apoA-I−/− mice (red hatched bars) treated with CSL-111 compared with vehicle-treated groups (F). The changes in body composition were due to reduced visceral fat mass in apoA-I+/+ mice (white hatched bars) (G) and due to reduced hepatic fat content in apoA-I−/− mice (red hatched bars) (H). Representative pictures of H&E-stained hepatic sections from WT and apoA-I −/− mice revealed accumulation of hepatic lipids in the form of lipid droplets within the hepatocytes in vehicle-treated apoA-I −/− mice (40× magnification), which normalized in the CSL-111 treatment group. CSL-111 treatment normalized cholesterol (J) and triglyceride (K) levels in the CNS of apoA-I−/− mice. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.00005 versus apoA-I+/+ mice. Atp5b, ATP synthase subunit β Cyc1, cytochrome C1; Mit2, mitofusin 2; Opa1, optic atrophy 1; Pgc1α, PPARc coactivator 1α.