Two closely related Synechococcus strains showed significantly different tolerances to high light and high temperature but limited genomic differences, providing us opportunities to identify key genes responsible for stress acclimation by a gene complementation approach. In this study, we confirmed that a single point mutation in the α subunit of FoF1 ATP synthase (AtpA) contributes mainly to the improved stress tolerance of Synechococcus elongatus UTEX 2973. The point mutation of AtpA, the important ATP-generating complex of photosynthesis, increases AtpA protein levels, intracellular ATP synthase activity, and ATP concentrations under heat stress, as well as photosystem II activity. This work proves the importance of ATP synthase in cyanobacterial stress acclimation and provides a good target for future improvement of cyanobacterial stress tolerance by metabolic engineering.
KEYWORDS: Synechococcus elongatus, ATP synthase, point mutation, abiotic stress, photosynthesis
ABSTRACT
In response to a broad range of habitats and environmental stresses, cyanobacteria have evolved various effective acclimation strategies, which will be helpful for improving the stress tolerances of photosynthetic organisms, including higher plants. Synechococcus elongatus UTEX 2973 and PCC 7942 possess genomes that are 99.8% identical but exhibit significant differences in cell growth and stress tolerance. In this study, we found that a single amino acid substitution at FoF1 ATP synthase subunit α (AtpA), C252Y, is the primary contributor to the improved stress tolerance of S. elongatus UTEX 2973. Site-saturation mutagenesis experiments showed that point mutations of cysteine 252 to any of the four conjugated amino acids could significantly improve the stress tolerance of S. elongatus PCC 7942. We further confirmed that the C252Y mutation increases AtpA protein levels, intracellular ATP synthase activity, intracellular ATP abundance, transcription of psbA genes (especially psbA2), photosystem II activity, and glycogen accumulation in S. elongatus PCC 7942. This work highlights the importance of AtpA in improving the stress tolerance of cyanobacteria and provides insight into how cyanobacteria evolve via point mutations in the face of environmental selection pressures.
IMPORTANCE Two closely related Synechococcus strains showed significantly different tolerances to high light and high temperature but limited genomic differences, providing us opportunities to identify key genes responsible for stress acclimation by a gene complementation approach. In this study, we confirmed that a single point mutation in the α subunit of FoF1 ATP synthase (AtpA) contributes mainly to the improved stress tolerance of Synechococcus elongatus UTEX 2973. The point mutation of AtpA, the important ATP-generating complex of photosynthesis, increases AtpA protein levels, intracellular ATP synthase activity, and ATP concentrations under heat stress, as well as photosystem II activity. This work proves the importance of ATP synthase in cyanobacterial stress acclimation and provides a good target for future improvement of cyanobacterial stress tolerance by metabolic engineering.
INTRODUCTION
As the most ancient oxygenic photosynthetic microorganisms, cyanobacteria have survived and emerged from multiple mass extinction events over the past 3.5 billion years (1). They are widely distributed in aquatic, terrestrial, and extreme habitats and are the numerically dominant global primary producers at present (2). Various effective adaptive strategies used by cyanobacteria to combat different environmental stresses have been explored and reported (3), and some of these have been successfully used for improving the stress tolerances of higher plants (4, 5). In recent years, cyanobacterial cells have been genetically engineered to produce many chemicals and fuels directly from carbon dioxide and solar energy by modifying natural metabolic pathways and installing heterologous synthetic pathways (6). Due to the complex environmental implications and serious contamination associated with outdoor scale-up cultivation, it is important to understand and improve the stress tolerance of cyanobacterial cell factories for future industrial applications.
Synechococcus elongatus UTEX 2973 (Sye2973) is a recently isolated fast-growing cyanobacterium with excellent stress tolerance (7), exhibiting its fastest photoautotrophic growth at 42°C and 1,500-μE m−2 s−1 illumination (8). In contrast, Synechococcus elongatus PCC 7942 (Sye7942) cannot grow under the same conditions (8). Interestingly, the genome sequences of these two Synechococcus strains are 99.8% identical. A total of 55 single nucleotide polymorphisms (SNPs) and insertions/deletions (indels) between these two genomes have led to amino acid changes in 21 chromosome-encoded and 3 plasmid-encoded proteins (7). Thus, some of these genomic differences may be responsible for the phenotypic differences between the two Synechococcus strains, particularly in terms of stress tolerance under high-temperature and high-light conditions. Among the proteins that differ in the two strains, chaperone DnaK, DNA-directed RNA polymerase, FoF1 ATP synthase, photosystem I (PSI) assembly protein Ycf4, and glutamate synthase may be related to significant differences in stress tolerance capability. However, there is no experimental evidence to predict which genes are involved and how they contribute to the improved performance of Sye2973 under stress conditions.
In cyanobacteria, photosynthesis involves a series of light-induced electron transfers from water to NADP+, accompanied by the acidification of the thylakoid lumen and the generation of oxygen, NADPH, and ATP (9). Moreover, photosynthesis is highly susceptible to environmental changes. Photosystem protection is one of the most important strategies for acclimation to abiotic stresses and involves (i) accelerating repairs of PSII, consisting in replacement of damaged D1 proteins with newly synthesized and matured ones (10), (ii) redistributing the excitation light energy between PSII and PSI (11, 12) and decreasing the light energy transferred to PSII through nonphotochemical quenching (NPQ) (13), (iii) increasing cyclic electron transport and decreasing linear electron transport, and (iv) regulating the transcription of stress-responsive genes encoding heat shock proteins (HSPs), superoxide dismutase (SOD), and the extrinsic PSII oxygen-evolving complex (3, 14).
In this study, a high-throughput plate-based screening method was newly developed to identify specific SNPs contributing to the high-temperature and high-light tolerance of Sye2973. Using this method, we identified a single SNP in atpA, encoding the FoF1 ATP synthase α subunit (AtpA), as the main contributor. This mutation, introduced into Sye7942, improved its stress tolerance, and a site-saturation mutagenesis experiment was performed to assess SNP plasticity. We demonstrated that point mutations of cysteine 252 to four conjugated amino acids could significantly improve the stress tolerance of Sye7942. Then we confirmed that the C252Y mutation in AtpA served to increase both the protein level and the intracellular activity of FoF1 ATP synthase, as well as increasing intracellular ATP abundance, psbA transcription, the PSII/PSI ratio, the linear electron transport rate, the oxygen evolution rate, and glycogen accumulation under heat stress conditions. These results elucidate the mechanism leading to the significant difference in stress tolerance between Sye2973 and Sye7942.
RESULTS
A single nonsynonymous SNP in the atpA gene is predominantly responsible for the improved stress tolerance of Sye2973.
To identify genes indispensable for the improved stress tolerance of Sye2973 over that of Sye7942 (Table 1), a plate-based screening method was established through gene complementation of Sye7942 by introducing different SNP-containing genes of Sye2973 (see Fig. S1A in the supplemental material) rather than constructing traditional gene knockouts (Fig. S1B) of Sye2973. It was found that Sye7942 cells loaded onto plates could not survive at 43°C and 220-μE m−2 s−1 illumination, whereas Sye2973 could survive not only under these conditions but also under even harsher conditions (43°C, 500-μE m−2 s−1 illumination) (see Fig. S2 in the supplemental material). Therefore, a two-step plate-based screening method was implemented for further transformation experiments, using 43°C and 220-μE m−2 s−1 illumination for the first round of screening and 43°C and 500-μE m−2 s−1 illumination for rechecking (see Fig. S3 in the supplemental material).
TABLE 1.
Bacterial strains used in this study
| Strain name | Genotype descriptiona | Source |
|---|---|---|
| Sye7942 | Synechococcus elongatus PCC 7942, wild type | Xudong Xu |
| Sye2973 | Synechococcus elongatus UTEX 2973, wild type | UTEXb |
| Sye7942-C252Y | Mutant strain of Sye7942 harboring AtpA with the C252Y point mutation | This study |
| Sye7942-C252H | Mutant strain of Sye7942 harboring AtpA with the C252H point mutation | This study |
| Sye7942-C252F | Mutant strain of Sye7942 harboring AtpA with the C252F point mutation | This study |
| Sye7942-C252W | Mutant strain of Sye7942 harboring AtpA with the C252W point mutation | This study |
AtpA, ATP synthase FoF1 subunit α.
UTEX, the Culture Collection of Algae at The University of Texas at Austin.
From an evolutionary perspective, genes containing nonsynonymous SNPs are more likely to lead to phenotypic changes than those harboring synonymous SNPs. As reported previously, there are 55 SNPs that cause nonsynonymous mutations in 24 proteins in Sye2973 (7). The small endogenous plasmid (GenBank accession number CP006473) harboring three nonsynonymous SNPs was found to be lost in the Sye2973 strain (Table 1) used in this study. Thus, recombinant plasmids (Table 2) harboring each of the 21 chromosome-encoded candidate genes from Sye2973 were used to transform Sye7942. After the two-step plate screening (Fig. S3 in the supplemental material), only high-temperature- and high-light-tolerant colonies could be isolated from the transformed cells of Sye7942. We found that all tolerant colonies isolated contained a SNP in the atpA gene, encoding the α subunit of FoF1 ATP synthase. The point mutation replacing cysteine 252 with tyrosine (C252Y) in AtpA was first confirmed by Sanger sequencing. This isolated mutant of Sye7942 was named Sye7942-C252Y, and like Sye2973, it was able to grow at 43°C and 500-μE m−2 s−1 illumination (Fig. 1A). Resequencing of the complete genome further ensured that this point mutation was the only mutation found in the genome of Sye7942-C252Y relative to the original wild-type strain of Sye7942. These findings confirmed that among 21 SNP-containing genes (Table 2), the atpA gene is the primary contributor to the increased stress tolerance of Sye2973.
TABLE 2.
Plasmids and primers used in this study
| Purpose and plasmid | Characteristicsa | Primers used for plasmid construction (5′ → 3′) |
|---|---|---|
| Identification of genes responsible for stress tolerance | ||
| pSK027 | Contains the M744_03965 gene, encoding ABC transporter substrate-binding protein; Apr Kmr | F1, GGGAATCCCCGCTAGCTTGGGCAACTGCAGC |
| R1, GTTGATGGCGGTGCGGAGCAGATCGGACAGG | ||
| pSK028 | Contains the M744_03975 gene, encoding a hypothetical protein; Apr Kmr | F2, CCTGTCCGATCTGCTCCGCACCGCCATCAAC |
| R2, CGGCAAGTAGGACTGCAGCTGCAAGCCAGCC | ||
| pSK029 | Contains the M744_04780 gene, encoding PpnK, inorganic polyphosphate/ATP-NAD kinase; Apr Kmr | F3, CGGCCAATTCTGTAGCGCGATCGATGACAG |
| R3, GGATCAGGAACCACCCTGATCCCCGCGAGC | ||
| pSK030 | Contains the M744_06025 gene, encoding molecular chaperone DnaK; Apr Kmr | F4, CCACAGTGCTGATGCCGCCGAATTGACCGC |
| R4, GGGCGAAGAAGCGGAGGCGATTCACAAGAGC | ||
| pSK031 | Contains the M744_06570 gene, encoding hydrolase; Apr Kmr | F5, GGGCACGGCTTGGGAAGGGCTGCAGGAATG |
| R5, CAGTCAGGCCACAGGCTGACATTCACACACC | ||
| pSK032 | Contains the M744_06650 gene, encoding CTP synthetase; Apr Kmr | F6, GGCGACAGTTCGCCCCGATTGTACTGCCTG |
| R6, GAGTGGTCGAATGCGAACGACTTGAAATCG | ||
| pSK033 | Contains the M744_05865 gene, encoding a hypothetical protein; Apr Kmr | F7, GGAATTACCTTTACTGAGCTTGAGCAATTAC |
| R7, CATTCGTGAGATTTTGATTATCTCCACCCC | ||
| pSK034 | Contains the M744_06850 gene, encoding chorismate mutase; Apr Kmr | F8, CTCCTAGCGGGGATTTTCTACAATCAGAAG |
| R8, CTTGCTCGCTGTAGCCACATTGCTCAGTAC | ||
| pSK035 | Contains the M744_11685 gene, encoding anthranilate synthase, component I; Apr Kmr | F9, CCCAACAACTGGCGGAGACTATCCCGAAAAG |
| R9, GAAAGTGAACGAAGGTCGCTCCTTCGCGGG | ||
| pSK036 | Contains the M744_12130 gene, encoding long-chain-fatty-acid CoA ligase; Apr Kmr | F10, GATCGCTTTGACATTGATCGTCCGCTTTAGC |
| R10, CAGGCTGAGACGAGCGATCGCCCAGTCG | ||
| pSK037 | Contains the M744_00705 gene, encoding a hypothetical protein; Apr Kmr | F11, GTTGTTGCAACAGCTGATTTTGTTGATCGAG |
| R11, CTAAGGCTGAGGATTGAGGCGCAGCGATCGC | ||
| pSK038 | Contains the M744_01335 gene, encoding ATP synthase FoF1 subunit α; Apr Kmr | F12, GAGAACTTGTTCTTGAGCCCGACGCACCTTG |
| R12, CAGCCAGAAAAAAGCGGTTCTCAACCAAGTG | ||
| pSK039 | Contains the M744_02605 gene, encoding chemotaxis protein CheY; Apr Kmr | F13, CATTTAATTGCCCTAGAAGATGCTGTTG |
| R13, CGATCACTGGTGGTGATTGAACAGGCTG | ||
| pSK040 | Contains the M744_02855 gene, encoding a pilus assembly chaperone; Apr Kmr | F14, GGATGAACTTGGTAACGTCTTGATCGAGTG |
| R14, CAGCAACGGCTCGGGTGTTGATGGAATTGG | ||
| pSK041 | Contains the M744_03320 gene, encoding 23S rRNA; Apr Kmr | F15, CATATTTGATAGAGCGGTATCAGAGGTCAAC |
| R15, CAGTACTACTCTCAAGCCTACCAACTGCCAG | ||
| pSK042 | Contains the M744_03335 gene, encoding manganese ABC transporter ATP-binding protein; Apr Kmr | F16, CTCTCAAGATTGCCGCTGAACTGTCGACACC |
| R16, GAATCAGCGACTGATAGTCTTCAAACAACTG | ||
| pSK043 | Contains the M744_03855 gene, encoding guanylate cyclase; Apr Kmr | F17, GGTCTACAGCAGATCGCTCACCGATCCATCG |
| R17, GAGTAGCCTGCGGCGCGGTCTGGATAAAACC | ||
| pSK044 | Contains the M744_08615 gene, encoding DNA-directed RNA polymerase subunit B; Apr Kmr | F18, CGGCGCTAAACGACCGAGGAGACGCATGGC |
| R18, CTTCAAAACCATCCCGCGATCACTTACGAC | ||
| pSK045 | Contains the M744_09095 gene, encoding porin; major outer membrane protein; Apr Kmr | F19, GCTTGCTGACAGGACTTTCATCATGGTGTG |
| R19, GTGACATTGCCCGCCCCATCCTGTTCTAGG | ||
| pSK046 | Contains the M744_12285 gene, encoding glutamate synthase; Apr Kmr | F20, CAGGCCATGGATGAGCTAACAGCGCAAAG |
| R20, GCTTTCTCCATTGGCCGGTTATCGCAGC | ||
| pSK047 | Contains the M744_13540 gene, encoding photosystem I assembly protein Ycf4; Apr Kmr | F21, GTTCGAAAACGCAATCCCGAAAATCTGGCTCC |
| R21, GCTGACCCACTCATGGGCGTTTGAGGTTGTC | ||
| In vivo site-saturation mutagenesis of AtpA | ||
| pSK090 | Contains the AtpA gene with the C252A mutation; Apr Kmr | 252A-1, GATCGCCGAGTACTTTATGGCTAAAGGCAAAGCC |
| 252A-2, AGCCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK091 | Contains the AtpA gene with the C252D mutation; Apr Kmr | 252D-1, GATCGCCGAGTACTTTATGGACAAAGGCAAAGCC |
| 252D-2, GTCCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK092 | Contains the AtpA gene with the C252E mutation; Apr Kmr | 252E-1, GATCGCCGAGTACTTTATGGAAAAAGGCAAAGCC |
| 252E-2, TTCCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK093 | Contains the AtpA gene with the C252F mutation; Apr Kmr | 252F-1, GATCGCCGAGTACTTTATGTTCAAAGGCAAAGCC |
| 252F-2, GAACATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK094 | Contains the AtpA gene with the C252G mutation; Apr Kmr | 252G-1, GATCGCCGAGTACTTTATGGGCAAAGGCAAAGCC |
| 252G-2, GCCCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK095 | Contains the AtpA gene with the C252H mutation; Apr Kmr | 252H-1, GATCGCCGAGTACTTTATGCACAAAGGCAAAGCC |
| 252H-2, GTGCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK096 | Contains the AtpA gene with the C252I mutation; Apr Kmr | 252I-1, GATCGCCGAGTACTTTATGATCAAAGGCAAAGCC |
| 252I-2, GATCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK097 | Contains the AtpA gene with the C252K mutation; Apr Kmr | 252K-1, GATCGCCGAGTACTTTATGAAAAAAGGCAAAGCC |
| 252K-2, TTTCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK098 | Contains the AtpA gene with the C252L mutation; Apr Kmr | 252L-1, GATCGCCGAGTACTTTATGCTGAAAGGCAAAGCC |
| 252L-2, CAGCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK099 | Contains the AtpA gene with the C252M mutation; Apr Kmr | 252M-1, GATCGCCGAGTACTTTATGATGAAAGGCAAAGCC |
| 252M-2, CTCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK100 | Contains the AtpA gene with the C252N mutation; Apr Kmr | 252N-1, GATCGCCGAGTACTTTATGAACAAAGGCAAAGCC |
| 252N-2, GTTCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK101 | Contains the AtpA gene with the C252P mutation; Apr Kmr | 252P-1, GATCGCCGAGTACTTTATGCCCAAAGGCAAAGCC |
| 252P-2, GGGCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK102 | Contains the AtpA gene with the C252Q mutation; Apr Kmr | 252Q-1, GATCGCCGAGTACTTTATGCAAAAAGGCAAAGCCG |
| 252Q-2, TTGCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK103 | Contains the AtpA gene with the C252R mutation; Apr Kmr | 252R-1, GATCGCCGAGTACTTTATGCGCAAAGGCAAAGCC |
| 252R-2, GCGCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK104 | Contains the AtpA gene with the C252S mutation; Apr Kmr | 252S-1, GATCGCCGAGTACTTTATGAGCAAAGGCAAAGCC |
| 252S-2, GCTCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK105 | Contains the AtpA gene with the C252T mutation; Apr Kmr | 252T-1, GATCGCCGAGTACTTTATGACCAAAGGCAAAGCC |
| 252T-2, GGTCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK106 | Contains the AtpA gene with the C252V mutation; Apr Kmr | 252V-1, GATCGCCGAGTACTTTATGGTGAAAGGCAAAGCC |
| 252V-2, CACCATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| pSK107 | Contains the AtpA gene with the C252W mutation; Apr Kmr | 252W-1, GATCGCCGAGTACTTTATGTGGAAAGGCAAAGCC |
| 252W-2, CCACATAAAGTACTCGGCGATCGCAGCACCGGC | ||
| Random library | Contains the AtpA gene with a random C252 mutation; Apr Kmr | 252random-1, GATCGCCGAGTACTTTATGNNNAAAGGCAAAGCC |
| 252random-2, NNNCATAAAGTACTCGGCGATCGCAGCACCGGC |
Ap, ampicillin; Km, kanamycin; AtpA, gene encoding the FoF1 ATP synthase α subunit.
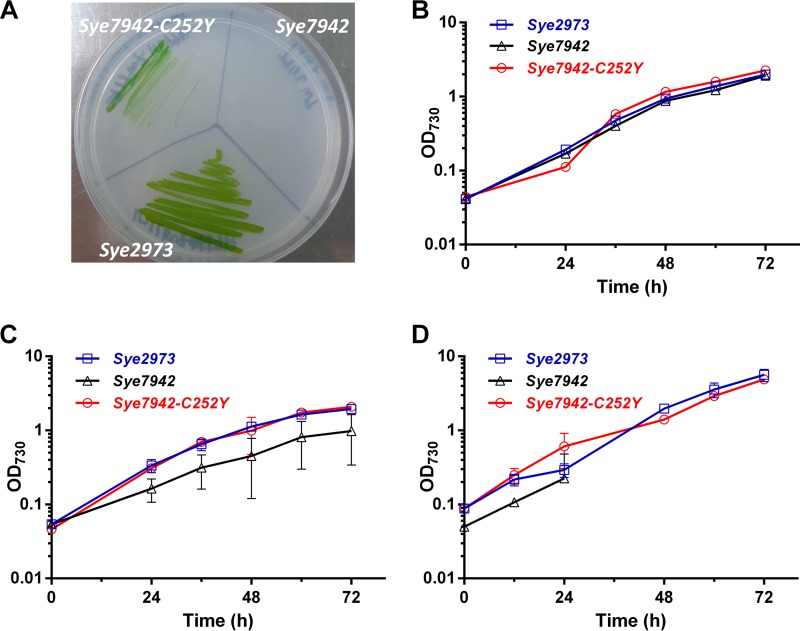
FIG 1.
Evaluation of stress tolerances of S. elongatus strains to different abiotic stresses. (A) S. elongatus strains grown on BG11 plates at 43°C with 500-μE m−2 s−1 illumination. (B through D) Growth of the same strains in liquid BG11 medium at 30°C with 50-μE m−2 s−1 illumination (B), at 41°C with 50-μE m−2 s−1 illumination (C), and at 41°C with 500-μE m−2 s−1 illumination (D). Error bars indicate standard deviations (n = 3).
The C252Y mutation in AtpA improves the tolerance of Sye7942 to abiotic stresses.
To systematically evaluate the tolerance of Sye7942-C252Y to abiotic stresses, its growth in liquid medium under normal conditions (Fig. 1B) and under high-temperature (Fig. 1C), high-light and high-temperature (Fig. 1D), and high-light (see Fig. S4 in the supplemental material) stress conditions was compared with that of wild-type Sye7942 and Sye2973. Under both normal (30°C, 50 μE m−2 s−1) (Fig. 1B) and high-light (Fig. S4) culture conditions, the growth of Sye7942-C252Y was similar to that of Sye2973 and Sye7942. When the temperature was increased to 41°C, however, the Sye7942-C252Y mutant clearly exhibited better growth than Sye7942 (Fig. 1C). Under high-light and high-temperature conditions (41°C, 500 μE m−2 s−1), the growth rate of Sye7942-C252Y increased significantly (P < 0.05), from 0.03 to 0.05 h−1 (Fig. 1D; see also Fig. S5 in the supplemental material), over that under normal conditions. Sye2973 showed a growth rate similar (0.04 h−1) to that of Sye7942-C252Y, while Sye7942 did not grow at all, under the same culture conditions (Fig. 1D; also Fig. S5). These results demonstrate that the C252Y point mutation in AtpA significantly improves the tolerance of Sye7942 to high-light and high-temperature stresses.
A point mutation of cysteine 252 to any of four amino acids with conjugated side chains improves the stress tolerance of Sye7942.
To further analyze the plasticity of this SNP, an artificial mutation selection approach was undertaken for AtpA evolution as follows. The C252 residue of AtpA was mutated in vivo to 19 other amino acids via random or specific mutation, and these mutations were screened using the screening method described above. After Sye7942 was transformed with a random plasmid library containing atpA genes with random codons (NNN) at amino acid position 252, the C252W, C252F, and C252H mutations in AtpA were observed among high-temperature- and high-light-tolerant colonies after phenotypic and genotypic confirmation (Table 3). Interestingly, all three of these amino acids and tyrosine together make up the only four conjugated amino acids in nature. Unexpectedly, the C252Y mutation was not found in this screening. This may be due to the small number (n = 35) of stress-tolerant colonies available for genotyping or to base composition bias in the plasmid library, which may have led to the lack of tyrosine-specific codons at position 252.
TABLE 3.
Codons and amino acids appearing at position 252 of AtpA from stress-tolerant colonies
| Amino acid expected | Codon used | Amino acid mutation | Codon confirmed by Sanger sequencing |
|---|---|---|---|
| Random | NNN | F | UUC |
| W | UGG | ||
| H | CAU | ||
| A | GCU | Y | UAC |
| D | GAC | ||
| E | GAA | ||
| F | UUC | F | UUC |
| G | GGC | F | UUC |
| H | CAC | H | CAC |
| I | AUC | ||
| K | AAA | Y | UAC |
| L | CUG | ||
| M | AUG | ||
| N | AAC | ||
| P | CCC | ||
| Q | CAA | ||
| R | CGC | F | UUC |
| S | AGC | ||
| T | ACC | ||
| V | GUG | ||
| W | UGG | W | UGG |
| Y | UAC | Y | UAC |
To ensure that all amino acid mutations were screened equally, 19 plasmids (Table 2), each harboring 1 of 19 specific C252 amino acid substitutions, were constructed and were used to transform Sye7942. Although stress-tolerant colonies could be isolated following transformation with eight plasmids, only four amino acid substitutions—C252W, C252F, C252H, and C252Y—were confirmed among the AtpA proteins in these colonies following genotyping (Table 3). Combined with the findings of random codon experiment mentioned above, our results indicate that only mutations of C252 in AtpA to conjugated amino acids (Y, W, F, H) can improve the tolerance of Sye7942 to high-temperature and high-light conditions. This implies that the conjugated side chain of the amino acid at position 252 in the ATP synthase α subunit might be crucial for the stress tolerance of Sye7942 under the conditions tested.
C252 point mutations increase AtpA protein levels, the intracellular enzymatic activity of FoF1 ATP synthase, and ATP levels.
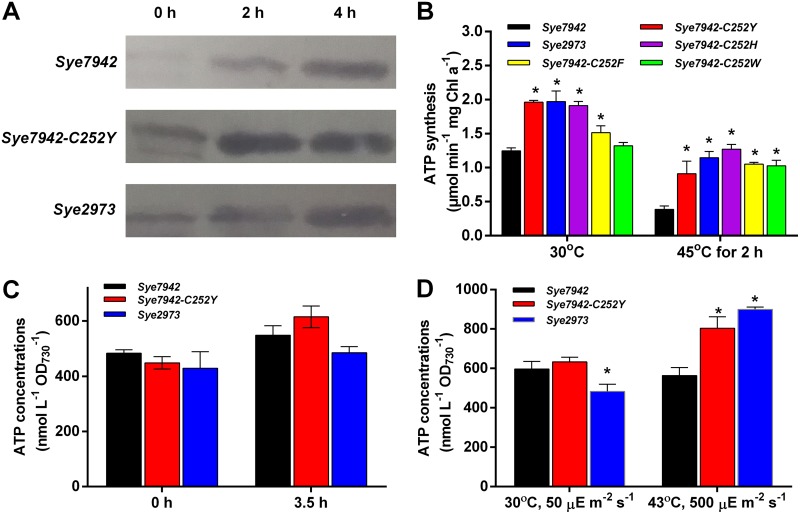
To determine how the C252Y mutation affects AtpA function, the AtpA expression levels, FoF1 ATP synthase activities, and intracellular ATP levels in crude extracts of different Synechococcus strains grown under both normal and heat stress conditions were determined and compared. As shown in Fig. 2A, AtpA levels were clearly elevated in all three strains under heat stress for 2 or 4 h. This increase in AtpA levels and the observed decrease in FoF1 ATPase synthase activity in Sye7942 during heat stress are consistent with previous findings (15, 16). Compared with Sye7942, both Sye7942-C252Y and Sye2973 clearly exhibited higher AtpA protein levels and FoF1 ATPase synthase activities under both normal and heat stress conditions (Fig. 2A and B). These results suggest that the C252Y mutation leads to increases in both AtpA accumulation and the FoF1 ATP synthase activity of Sye7942 under both normal and heat stress conditions. Furthermore, the FoF1 ATPase synthase activities of three other AtpA mutants, Sye7942-C252H, Sye7942-C252F, and Sye7942-C252W, under heat stress conditions (Fig. 2B) were also significantly higher than that of the wild-type Sye7942 strain (P < 0.05). The results prove that the mutation of C252 to any of these four conjugated amino acids (Y, W, F, H) could improve intracellular FoF1 ATPase synthase activity under heat stress conditions. This strengthened ATP synthesis was supposed to increase intracellular ATP abundance in Synechococcus. As shown in Fig. 2C, the ATP concentration in Sye7942-C252Y cells under the heat shock condition was higher than that in Sye7942 (P > 0.05), as expected. Additionally, both Sye7942-C252Y and Sye2973 cells contained more ATP when grown under high-light and high-temperature conditions (P < 0.05) (Fig. 2D). These results suggest that the C252Y mutation could enhance the intracellular ATP contents of Sye7942 under stress conditions.
FIG 2.
Effects of heat and high-light stresses on AtpA protein levels, FoF1 ATP synthase activity, and intracellular ATP contents. (A) Determination of AtpA protein levels under both normal and heat shock conditions by Western blotting. Five micrograms of each protein sample was used for Western blotting. To show the normalization of protein samples, SDS-PAGE analysis of the same samples was conducted (Fig. S9 in the supplemental material). (B) FoF1 ATP synthase activities of S. elongatus strains cultured under normal conditions and at 45°C for 2 h. (C) Determination of ATP contents in S. elongatus strains cultured under normal and heat shock conditions. Log-phase cultures of three Synechococcus strains were transferred to 45°C and were incubated at 45°C for 3.5 h. The ATP contents in these strains with or without heat shock were determined. (D) S. elongatus strains were inoculated at an initial OD730 of ∼0.05 and were grown under normal conditions (30°C, 50 μE m−2 s−1) or high-light and high-temperature conditions (43°C, 500 μE m−2 s−1) for 30 h. The ATP contents in these strains were then determined and compared. Stars indicate significant differences (P < 0.05) between Sye7942 and other strains grown under the same culture condition. Error bars indicate standard deviations (n = 3).
C252Y mutation enhances the photosynthetic efficiency of Sye7942.
According to previous reports, the ATP supply, controlled by FoF1 ATP synthase, regulates the turnover rate of the PSII core protein D1 during the acclimation of cyanobacteria to stress (10). A higher turnover rate of the D1 protein might further affect the photosynthetic efficiency of Synechococcus. Thus, multiple photosynthetic parameters, including the excitation energy distribution between PSII and PSI, NPQ, electron transport rate (ETR), and oxygen evolution rate, were measured and analyzed in both Sye7942 and Sye7942-C252Y.
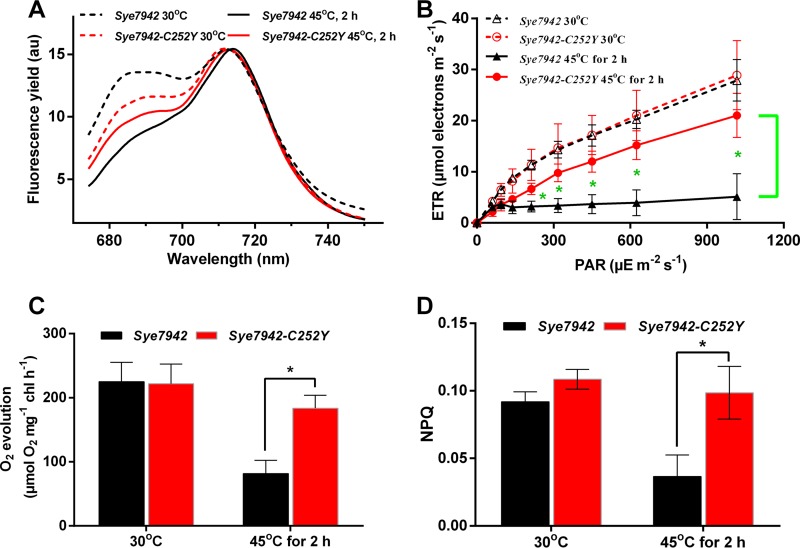
In the fluorescence emission spectra at 77 K shown in Fig. 3A, the PSII/PSI ratios in both Sye7942 and Sye7942-C252Y were lower under heat stress than under normal conditions. Moreover, PSI turnover was found to be significantly higher in all strains under the high-temperature and high-illumination condition (41°C, 200 μE m−2 s−1) than under the normal condition (see Table S1 in the supplemental material). Sye7942-C252Y also showed a significantly higher PSI turnover rate at 38°C than at 30°C. These results indicate that in response to heat shock, less light energy is transferred to the PSII reaction center to decrease photodamage to the PSII reaction center, and cyclic electron transport is used to generate more ATP in Synechococcus cells. The Sye7942-C252Y mutant showed a PSII/PSI ratio of 0.71, which was 3% higher (P > 0.05) than that in Sye7942 (0.69), under heat stress (Fig. 3A). This may result in a higher linear electron transport rate and increased PSII activity. As expected, both the ETR and the oxygen evolution rate were found to be significantly higher in Sye7942-C252Y (4.88- and 1.17-fold, respectively) than in Sye7942 under heat stress conditions (Fig. 3B and C). Moreover, there was no significant difference (P > 0.05) in the cyclic electron transport rate between Sye7942 and Sye7942-C252Y (Table S1). These results confirm that the Sye7942-C252Y mutant exhibits higher PSII activity than Sye7942 under heat stress. Besides regulating the light energy distribution between PSII and PSI, the NPQ mechanism is generally used by cyanobacteria to decrease the light energy transferred to the PSII reaction center; this depends mainly on an IsiA-related mechanism in Sye7942, which is generally and largely upregulated under stress conditions (17). After heat shock, the NPQ value of Sye7942 was decreased by about 50%, while that of Sye7942-C252Y was maintained at the same level as that under normal conditions (Fig. 3D). These results show that Sye7942-C252Y has a more reliable photoprotection system (NPQ) than Sye7942 in response to photodamage.
FIG 3.
Effects of heat shock on photosynthesis in Sye7942 and Sye7942-C252Y. Both Synechococcus cultures were grown at 30°C and 50 μE m−2 s−1 to the log phase, shifted to 45°C and 50 μE m−2 s−1, and then incubated for another 2 h. The 77 K fluorescence emission spectra (excitation at 430 nm) (A), electron transport rate (ETR) (B), oxygen evolution rate (C), and nonphotochemical quenching (NPQ) value (D) were determined for Sye7942 and Sye7942-C252Y with or without a heat shock at 45°C for 2 h. 77 K fluorescence emission spectra were recorded from 674.2 to 750.2 nm, with the PSII peak at 695 nm and the PSI peak at 715 nm. F695/F715, which was calculated by dividing the fluorescence intensity at 695 nm (F695) by that at 715 nm (F715), was used as a relative indicator of the distribution of excitation energy between PSII and PSI (PSII/PSI ratio). Stars indicate significant differences (P < 0.05) in photosynthetic parameters between Sye7942 and Sye7942-C252Y grown under the same culture conditions.
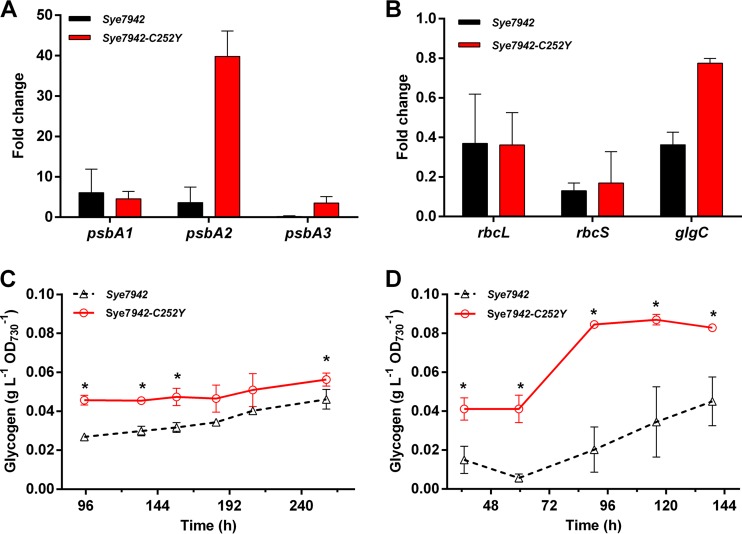
To examine the effects of the C252Y mutation on the photorepair process, the transcript abundances of three psbA genes during heat shock were determined by quantitative real-time reverse transcription-PCR (qRT-PCR) approaches. After 2 h of heat stress, the psbA1 transcript levels of strain Sye7942 were 5.1-fold higher than those under normal conditions. Similar upregulation of psbA1 was observed in strain Sye7942-C252Y. The psbA2 transcript levels of strain Sye7942 were increased 2.6-fold, while the same gene was upregulated 38.8-fold in strain Sye7942-C252Y, after heat shock. In contrast, under heat stress, psbA3 transcript levels were significantly lower in the wild-type strain Sye7942, but 3.5-fold higher in Sye7942-C252Y, than those under normal conditions (Fig. 4A). Considering that psbA2 and psbA3 encode form II of the D1 protein, D1:2 (18), these results suggest that the C252Y mutation increases the percentages of the D1:2 proteins in the PSII reaction center of Sye7942-C252Y and accelerates replacement of the damaged D1 protein in response to heat stress, relative to those in Sye7942.
FIG 4.
Effects of the C252Y mutation on psbA transcription and photosynthetic carbon fixation. Changes in gene transcript levels of Synechococcus strains shocked by heat (45°C) for 2 h were evaluated and compared by real-time PCR. The fold change was calculated as 2−[(CT_gene_45°C − CT_ref_45°C) − (CT_gene_30°C − CT_ref_30°C)], where CT_gene_45°C, CT_ref_45°C, CT_gene_30°C, and CT_ref_30°C refer to the CT values of the tested gene at 45°C, the reference gene (rnpB) at 45°C, the tested gene at 30°C, and the reference gene (rnpB) at 30°C, respectively. (A) D1 protein-encoding genes (psbA1, psbA2, and psbA3). (B) Genes encoding subunits of RuBisCO (rbcL and rbcS) and ADP-glucose pyrophosphorylase (glgC). (C and D) Glycogen contents of Sye7942 and Sye7942-C252Y grown under normal (30°C, 50 μE m−2 s−1) (C) and high-temperature (41°C, 50 μE m−2 s−1) (D) conditions. Stars indicate significant differences (P < 0.05) in gene transcription or glycogen content between Sye7942 and Sye7942-C252Y grown under the same culture conditions.
Analysis of all these photosynthetic parameters suggests that the strengthened ATP synthesis caused by the C252Y mutation increases the turnover rate of the D1 protein and enhances PSII activity and the NPQ system under heat stress, which accounts for the improved stress tolerance of Sye7942-C252Y.
C252Y mutation enhances photosynthetic carbon fixation.
To evaluate the effects of the C252Y mutation in AtpA on photosynthetic carbon fixation, the transcript levels of some key genes encoding subunits of RuBisCO and ADP-glucose pyrophosphorylase in Sye7942 and Sye7942-C252Y were determined and compared through qRT-PCR approaches. Under heat stress, rbcL and rbcS, encoding two subunits of RuBisCO, and glgC, encoding ADP-glucose pyrophosphorylase, were repressed in Sye7942 (Fig. 4B). In Sye7942-C252Y, transcript levels of both rbcL and rbcS were not significantly different from those in Sye7942 (Fig. 4B).
Glycogen is the major carbon storage source in photosynthetic cyanobacteria (19). To further evaluate the effects of the C252Y mutation on glycogen accumulation, both the wild-type strain Sye7942 and the Sye7942-C252Y mutant were grown under normal and stress conditions, and their glycogen contents were analyzed and compared. Under normal culture conditions (30°C, 50 μE m−2 s−1), the glycogen content of strain Sye7942-C252Y was about 0.05 g liter−1 OD730−1, which is almost 1.2- to 1.7-fold that of the wild-type strain (Fig. 4C). When these strains were grown under high-temperature stress conditions (41°C, 50 μE m−2 s−1) for >90 h, the glycogen content of strain Sye7942-C252Y was increased to 0.08 g liter−1 OD730−1, significantly higher than that of the wild-type strain (Fig. 4D). These results prove that the C252Y mutation significantly promotes intracellular glycogen accumulation under both normal and heat stress conditions.
AtpA is highly conserved in cyanobacteria.
AtpA is a highly conserved protein among cyanobacterial species (see Table S2 in the supplemental material). Of 353 cyanobacterial AtpA homologs, 88.95% were found to have a conserved tyrosine at the 252nd amino acid position, whereas the AtpA protein of Sye7942 is the only one containing a cysteine at the same position (see Fig. S6a and Table S2 in the supplemental material). By phylogenetic analysis, the AtpA sequences from most Synechococcus species, including Sye7942 and Sye2973, as well as all Prochlorococcus species, were found to be clustered together (Fig. S6b and S7). However, the AtpA sequences of Sye7942 and Sye2973, as well as other sequences in this clade, clearly evolved from their common ancestor into two divergent groups (Fig. S6b and S7). Interestingly, both Sye7942 and Sye2973 were isolated from freshwater, while most of the other species analyzed were from marine or brackish environments (see Table S3 in the supplemental material). These findings indicate that the AtpA proteins from Sye7942 and Sye2973 show different evolution directions from their homologs in other Synechococcus species living in diverse environments.
DISCUSSION
As the cyanobacterial isolate with the highest known growth rate, Sye2973 has shown great potential for biochemical production and has been considered a promising photosynthetic cell factory (20). Its rapid growth under high-light and high-temperature conditions, and the limited genomic differences between Sye7942 and Sye2973 (7), provided us with an opportunity to gain insight into the latter strain's special stress acclimation mechanisms. In this study, we confirmed that, uniquely among single amino acid substitutions in 21 proteins, the C252Y mutation in the α subunit of the FoF1 ATP synthase protein (AtpA) significantly increases AtpA protein levels, intracellular FoF1 ATP synthase activity, intracellular ATP contents, and psbA transcripts encoding the D1 protein (especially the D1:2 protein), enhances PSII activity, and, finally, results in the promotion of stress tolerance and glycogen accumulation in Sye7942.
FoF1 ATP synthase has been demonstrated to modulate various important physiological activities in higher plants, including NPQ of excitation energy (21), photosynthetic electron transport and carbon fixation (22), and abiotic stress tolerance (23). Moreover, the relationships among the intracellular ATP supply, PSII repair, and environmental stresses have been investigated in detail in cyanobacteria and have been summarized in a recent review (24). In brief, PSII is inevitably damaged during photosynthesis, while environmental stresses do not affect PSII photodamage, or affect it only slightly, but inhibit PSII repair (25, 26). ATP is required for most of the steps of PSII repair (10, 24), such as the degradation of damaged D1, transcription of psbA genes, synthesis and maturation of D1, and reassembly of the PSII complex. Also, overproduction of the D1:2 protein has been proven to make Synechococcus cells more tolerant to photoinhibition of PSII under stress conditions (27). Based on our observations and these previous reports, we hypothesize that the increased FoF1 ATP synthase activity in Synechococcus caused by the C252Y mutation first improves the intracellular energy supply, accelerates the ATP-dependent repair of the D1 protein, changes the proportion of the D1:2 protein, and recovers inhibited photosynthetic activity; then photosynthesis powers more energy-dependent processes, including NPQ, as well as carbon fixation pathways, to further protect cells from stresses and accelerate cell growth. Our findings demonstrate the roles of AtpA in photosynthesis and stress tolerance in cyanobacteria and indicate that AtpA might be a promising candidate for improving stress tolerance in other photosynthetic systems, such as other cyanobacteria, microalgae, and even plants.
Through homology modeling analysis, it is clear that cysteine 252 is located neither in the active center nor at the interaction surface between the α and β subunits but rather on the surface of the whole F1 complex (see Fig. S8 in the supplemental material). Thus, the C252Y mutation seems to have no effects on neighboring residues but instead affects protein activity or protein-protein interactions. This mutation could disrupt or induce protein-protein interactions between AtpA and some chaperones, such as Atp23 (28), Atp11/12 (29), and FMC1 (30) in yeast and HSP90 (31) in all eukaryotes, which might stabilize and affect the activity of FoF1 ATP synthase.
The fact that most cyanobacterial species have a conserved tyrosine at the 252nd amino acid position raised another question: why some cyanobacterial species harboring Y252 in their AtpA proteins (for instance, Synechocystis sp. strain PCC 6803) do not show stress tolerances as high as that of Sye2973. This could be explained through the different genetic backgrounds of Sye2973 and these other cyanobacterial species.
According to a previous report (7), Sye2973 is a high-temperature-tolerant mutant of S. elongatus UTEX 625; the latter showed good tolerance to 38°C in the beginning but lost this ability over 6 decades of culturing in labs. Similarly, RpoS is rarely inactivated in natural Escherichia coli isolates within their mammalian hosts but is frequently disrupted during stab storage in labs. Considering these reports, we hypothesize that a similar microevolution event took place in Sye7942. Sye7942 AtpA might have had Y252, just like most of its homologs, when this strain (also known as Anacystis nidulans R2) was isolated from wild environments. Then the Y252C mutation in AtpA might have appeared during serial subculturing for decades under lab culture conditions, which routinely use 30°C and weak illumination. And Sye7942 is speculated to have finally lost its original high stress tolerance without facing stress pressures for decades due to the point mutation of AtpA.
How did cyanobacteria, a group of ancient microorganisms, survive the huge environmental changes over the past 3.5 billion years of evolutionary history? The effective stress acclimation strategies observed in this study and previous work provide part of the explanation. In fact, the dynamic nature of cyanobacterial genomes is also an important reason. Besides spontaneous mutations (32), most cyanobacteria are naturally competent (33–35) or able to be transformed by conjugation (36). Thus, they can take up DNA molecules from the living environment and easily integrate them into their own genomes by homologous recombination. In addition, the presence of multiple copies of chromosomes (37) provides cyanobacteria with the ability to tolerate certain harmful mutations. Mutations benefiting the cyanobacterial host are selected and fixed in the genome of the host under selective pressures (32). The transformation, site-saturation mutagenesis, and screening experiments that we performed in this study have illustrated how cyanobacteria might have evolved via a single amino acid point mutation in the face of environmental selection pressures.
Our work here highlights the importance of ATP synthase in the environmental stress tolerance of cyanobacteria, and it demonstrates the plasticity of genetic mutations for stress acclimation. Generally, our findings will better our understanding of stress acclimation mechanisms and be valuable for the improvement of the stress tolerance of photosynthetic organisms, including cyanobacteria and higher plants.
MATERIALS AND METHODS
Strains and culture conditions.
E. coli strain DH5α (TaKaRa, Dalian, China) was used for cloning. Sye7942 was from Xudong Xu's lab at the Institute of Hydrobiology, Chinese Academy of Sciences. Sye2973 was purchased from the Culture Collection of Algae at The University of Texas at Austin (UTEX). The strains constructed and used in this study are listed in Table 1. Sye7942 and Sye2973 were cultured in BG11 medium (38) at 30°C under 3% CO2, under continuous illumination (50 μE m−2 s−1); these are defined as the normal culture conditions in this work. The growth of each cyanobacterial strain was monitored by measuring the optical density at 730 nm (OD730).
For evaluating the stress tolerance of Synechococcus strains, Synechococcus cultures were inoculated into column photobioreactors (39) containing 200 ml liquid BG11 medium with an initial OD730 of 0.05 and were then grown under normal conditions (30°C, 50 μE m−2 s−1) or high-light (30°C, 500 μE m−2 s−1), high-temperature (41°C, 50 μE m−2 s−1), or high-light and high-temperature (41°C, 500 μE m−2 s−1) stress conditions. All cultures were bubbled with 3% CO2.
For the determination of AtpA protein levels, FoF1 ATP synthase activities, and gene transcript levels, Synechococcus cultures were inoculated at an initial OD730 of 0.2, grown under normal conditions (30°C, 50 μE m−2 s−1, 3% CO2) for 48 h, and then incubated at 45°C, 50-μE m−2 s−1 illumination, and 3% CO2 aeration for 2 or 4 h.
For the determination of intracellular glycogen contents, Synechococcus strains were inoculated into column photobioreactors (39) containing 200 ml liquid BG11 medium with an initial OD730 of 0.05 and were then grown under normal (30°C, 50 μE m−2 s−1) and high-temperature stress (41°C, 50 μE m−2 s−1) conditions. All cultures were bubbled with 3% CO2.
Plasmid and strain construction.
For the identification of genes responsible for stress tolerance, 21 protein-encoding genes containing SNPs between Sye7942 and Sye2973 were cloned from the chromosome of Sye2973 via PCR using the primers listed in Table 2. PCR products were purified using the E.Z.N.A. Cycle Pure kit (Omega Bio-tek, Norcross, GA, USA) and were ligated into the pEASY-Blunt vector (TransGen Biotech, Beijing, China). The resulting plasmids were then verified by Sanger gene sequencing and are listed in Table 2. For in vivo site-saturation mutagenesis of AtpA, pSK038 was used as the template for the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The primers are listed in Table 2, and the resulting plasmids were confirmed by Sanger DNA sequencing and are listed in Table 2.
Transformation of Sye7942.
Sye7942 was transformed with a recombinant plasmid according to a previously reported method (35) with some modifications. Briefly, 500 μl wild-type Sye7942 with an OD730 of 0.5 to 1.0 was centrifuged at 6,000 × g for 2 min and was then resuspended in 200 μl of fresh BG11 medium. Next, 200 ng of each plasmid was added, and the mixture was incubated in the dark at 30°C overnight. For two-step plate screening (Table S3 in the supplemental material), DNA-Synechococcus mixtures were spread on solid BG11 plates with 1.5% agar after transformation and were incubated at 43°C and 220-μE m−2 s−1 illumination for 3 days. Then transformants were picked, streaked on BG11 plates, and incubated at 43°C and 500-μE m−2 s−1 illumination for 3 days. Wild-type Sye7942 and Sye2973 were used as negative and positive controls, respectively. Transformants that survived after the two-step screening were considered true high-light- and high-temperature-tolerant colonies for subsequent genotyping experiments. The SNP-containing DNA fragments were amplified from the genomic DNA of these transformants for Sanger sequencing. The AtpA point mutants of Sye7942 were rescreened at 43°C and 500-μE m−2 s−1 illumination for 3 days until the sequencing results indicated that all copies of the gene were mutated. Only homozygous mutants were used in the subsequent physiological and metabolic analyses.
Sequence analysis.
For sequence alignment of the AtpA protein, the amino acid sequences of AtpA were retrieved by using blastP against the nonredundant protein database with the AtpA sequence from Sye7942, and sequences with >90% coverage and >49% identity were selected for the subsequent multiple-sequence alignment, performed with BioEdit (40). Logos were generated using the WebLogo server (41) with the multiple-sequence alignment mentioned above as an input. For phylogenetic analysis, an unrooted neighbor-joining tree was created in MEGA6 (42) using the default parameters. Bootstrap analysis was performed using 1,000 replicates.
For whole-genome resequencing of Sye7942-C252Y, genomic DNA was isolated, fragmented, blunted, modified with a 3′-A overhang, ligated to Illumina's standard sequencing adapters, and amplified by PCR. The library was sequenced as paired-end reads using an Illumina HiSeq 2500 sequencer. Library construction and sequencing were performed by Allwegene Tech (Beijing, China). The data were deposited in the NCBI Sequence Read Archive (SRA) under accession number SRR6246213.
The genome and plasmid sequences of Sye7942 were obtained from GenBank (accession numbers NC_007595.1, NC_004073.2, and NC_004990.1) and were used as reference sequences for read mapping. BWA (43) was used for mapping reads to reference sequences, and SAMtools (44) was used to identify SNPs between reference sequences and our data. The SNP-containing DNA fragments detected were amplified from the genomic DNA of both Sye7942 and Sye7942-C252Y and were verified by Sanger sequencing. Only the nucleotide mutation appearing in Sye7942-C252Y and not in Sye7942 was considered the true SNP.
Preparation of membrane vesicles.
Cells were grown from an initial OD730 of 0.2 to 1.2 under normal conditions and were then exposed to 45°C for 2 or 4 h. The method used has been described in a previous report (45). The cells were collected at 6,000 × g for 10 min at 4°C and were suspended in precooling buffer A (1.0 M betaine, 0.4 M d-sorbitol, 20 mM Tris-HCl [pH 7.0], 15 mM CaCl2, 15 mM MgCl2, 1 mM 6-amino-n-caproic acid, 1 mM phenylmethylsulfonyl fluoride [PMSF]). Then the cells were lysed by two passages through a French pressure cell (Constant Systems Limited, Daventry, UK) at a pressure of 2 × 107 Pa. The homogenate was centrifuged at 4,000 × g for 20 min at 4°C to remove the unbroken cells and cell debris. The thylakoid membrane was collected by centrifugation at 20,000 × g for 1 h at 4°C and was then resuspended in buffer A. The thylakoid membrane preparations were frozen in liquid nitrogen and were stored at −80°C.
Determination of the activity of FoF1 ATP synthase.
The acid/base transition method was used to determine the activity of FoF1 synthase at 25°C, as described in a previous report (46). Thylakoid membrane vesicles were incubated in buffer B (acid medium, containing 23 mM succinic acid, 6.7 mM MgC12, 2 mM KCl, 50 mM NaCl, 10 mM Tris-HCl [pH 6.0], 5 mM NaH2PO4, 1.4 pM valinomycin, 10 pM diuron, and 3.75 mM dithiothreitol) for 1 min at a concentration of 20 μg chlorophyll/ml. Then the suspension was diluted 1:1 with buffer C (6.7 mM MgCl2, 2 mM KCl, 50 mM NaCl, 200 mM Tris-HCl, 5 mM dithiothreitol, and 2 mM ADP) and was incubated for 30 s. The reaction in the 200-μl reaction mixture was stopped by boiling for 5 min. The ATP concentration was analyzed by a luciferase luminescence assay (Beyotime, Haimen, China). The activity of FoF1 ATPase synthase was calculated according to the ATP concentration, the reaction time, and the chlorophyll content.
Determination of intracellular ATP contents.
Intracellular concentrations of ATP were determined and calculated with an ADP/ATP ratio bioluminescence assay kit (K255-200; BioVision) according to the instructions.
Immunoblot analysis.
An anti-AtpA serum was generated by immunizing rabbits with the synthetic oligopeptide CLKTSKPEFIEKVQS. The total protein extracted from Sye7942, Sye7942-C252Y, and Sye2973 was quantified using the Bradford method (47), and equal amounts (5 μg) of protein were resolved by SDS-PAGE with a 12% sodium dodecyl sulfate-polyacrylamide gel (see Fig. S9 in the supplemental material). Proteins were then blotted onto nitrocellulose membranes and were detected with the anti-AtpA antibody.
Photosynthetic parameter determination.
Rapid light response curves and fluorescence induction measurements were determined using a Water-PAM (water pulse-amplitude-modulated) chlorophyll fluorometer (Walz GmbH, Effeltrich, Germany) after 15 min of dark adaptation. An actinic light intensity of 61 to 1,017 μE m−2 s−1 was used in the rapid light response curves. The fluorescence yields F′ and Fm′ were determined before or after the last saturating light pulse (800 ms, 3,000 μE m−2 s−1), respectively, at the end of each actinic illumination period. The ETR was calculated using the formula PAR × (Fm′ − F′)/Fm′, where PAR (photosynthetically active radiation) is the actinic light intensity (48, 49). The fluorescence induction measurements were performed under 61-μE m−2 s−1 continuous actinic light. At the same time, saturating flashes (800 ms, 3,000 μE m−2 s−1) were provided every minute until the maximal fluorescence yield (Fm′, for the light-adapted samples) was stable. After the measurements, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) was added to the samples at a final concentration of 20 μM. Then the maximal fluorescence yield (Fm DCMU) was measured under the actinic light and saturating flash (800 ms, 3,000 μE m−2 s−1). The NPQ was calculated as (Fm DCMU − Fm′)/Fm′.
The photosynthetic oxygen evolution rate was measured by a Clark-type oxygen electrode (DW1; Hansatech, Norfolk, UK). The temperature was controlled by a thermostatic water bath at 30°C and 45°C, and the light intensity was 625 μE m−2 s−1. The cells were collected by centrifugation at 4,000 × g for 5 min, resuspended in fresh BG11 medium supplemented with 1 mM KHCO3, and incubated under environmental conditions for 20 min. The photosynthetic oxygen evolution rate was calculated according to the chlorophyll content, which was determined as described in previous studies (50).
Low-temperature fluorescence (77 K fluorescence emission spectra) was measured with a Hitachi F-4500 fluorescence spectrophotometer (Hitachi High-Technologies Co., Tokyo, Japan) as described in a previous report (51). Samples were prepared at a concentration of 4 μg chlorophyll ml−1 and were immediately frozen in liquid nitrogen. The excitation wavelength was 430 nm, and the emission spectra were recorded from 674.2 to 750.2 nm, with bandwidths of 10 nm and 5 nm, respectively. The intensity of fluorescence emission at 695 nm (F695), arising from PSII, was divided by the intensity of emission at 715 nm (F715), arising from PSI. F695/F715 was used as a relative indicator of the distribution of excitation energy between PSII and PSI (PSII/PSI ratio) (11).
P700 reduction kinetics were determined as described previously (52) using a Joliot-type spectrophotometer (JTS-10; Bio-Logic, Grenoble, France). DCMU was added to the samples at a final concentration of 20 μM. The excitation wavelength was 630 nm (320 μE m−2 s−1), and the determination wavelengths were 705 nm and 750 nm. The turnover rate of PSI, in electrons per second (K), was calculated according to the formula K = 0.693/half-time of P700 reduction (in seconds).
qRT-PCR.
Both Sye7942 and Sye7942-C252Y were grown under normal conditions to the exponential phase and were shocked at 45°C for 2 h. Cultures with or without heat shock were collected and quickly frozen in liquid nitrogen. RNA extraction and quantitative real-time reverse transcription-PCR (qRT-PCR) were performed as described previously (53, 54). The primers are listed in Table 4.
TABLE 4.
Primers for real-time PCR analysis
| Gene | Primer designation | Primer sequence |
|---|---|---|
| rbcL | Synpcc7942_1426-1 | AATCCACAAATCGCAAGCA |
| Synpcc7942_1426-2 | TGAAACCAGCCGTCAAGAA | |
| rbcS | Synpcc7942_1427-1 | GGAAGAGTTCTACTGGACGAT |
| Synpcc7942_1427-2 | TTGGCACTGCTTGATGTT | |
| glgC | Synpcc7942_0603-1 | ACTCTGCCTCGCTCAACC |
| Synpcc7942_0603-2 | CTCATCCACATCCCACTCTT | |
| psbA1 | psbA1-1 | GCCCTTTACAACCTCAAGAT |
| psbA1-2 | ATCATCAGCACGCCGAAC | |
| psbA2 | psbA2-1 | ATTAAGTCTCGTAAATAGTTCAACT |
| psbA2-2 | TGGTTACCCACTCGCAGAA | |
| psbA3 | psbA3-1 | GGCATTACGCCTTAGACATAC |
| psbA3-2 | CGGTGCTGGTTACCCACT | |
| rnpB | rnpB-1 | AGCAAGGTGGAGGGACAA |
| rnpB-2 | CGAAGACAGAGGGCAGTTAT |
Glycogen content determination.
For the determination of cellular glycogen contents, Synechococcus cells were collected at 12,000 × g for 10 min at 4°C, washed twice with sterile water, suspended in 30% (wt/vol) KOH, and incubated at 95°C for 2 h. Ethanol was added to the suspension at a final concentration of 75% (vol/vol), with subsequent incubation at −20°C for 2 h to precipitate glycogen. Glycogen was collected by centrifugation (12,000 × g, 10 min, 4°C), washed with 70% and 98% ethanol in turn, and dried in a SpeedVac concentrator (Eppendorf, Germany). The dried glycogen was resuspended in 100 mM sodium acetate (pH 4.5) and was enzymatically hydrolyzed to glucose by amyloglucosidase (Novozymes, Denmark) at 60°C for 2 h. The glycogen content was determined by monitoring the glucose concentration using a biosensor (SBA-40C; Shandong Academy of Sciences, China).
Statistical analysis.
The values shown in graphs are expressed as means ± standard deviations. The significance of differences between groups was determined by t tests using IBM SPSS Statistics, version 19 (SPSS, Chicago, IL, USA). A P value of <0.05 was considered to indicate significance.
Accession number(s).
All whole-genome sequencing data that support the findings of this study have been deposited in the National Center for Biotechnology Information Sequence Read Archive (SRA) with accession number SRR6246213.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bao-Sheng Qiu for technical assistance with the analysis of photosynthesis parameters. We thank Yajing Liang for assistance with protein structure analysis.
This work was supported by the National Science Fund for Distinguished Young Scholars of China (award 31525002 to X.L.), Shandong Key Basic Research Project (to X.L.), the Key Research Program of the Chinese Academy of Sciences (award ZDRW-ZS-2016-3 to X.T.), the National Science Foundation of China (award 31301018 to X.T.; award 31600034 to G.L.), the Shandong Taishan Scholarship (X.L.), the Qingdao Innovative Leading Talent project [award 15-10-3-15-(31)-zch], and Scientific Funds for Outstanding Young Scientists of Shandong Province (award BS2014HZ003 to C.L.).
We declare no conflicts of interest regarding the contents of this article.
X.L. and X.T. conceived and supervised the study. W.L., X.T., K.S., S.Z., and C.L. performed the experiments and analyzed the data. X.T., W.L., K.S., G.L., and X.L. wrote the report.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01222-18.
REFERENCES
- 1.Blankenship RE. 2010. Early evolution of photosynthesis. Plant Physiol 154:434–438. doi: 10.1104/pp.110.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomitani A, Knoll AH, Cavanaugh CM, Ohno T. 2006. The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc Natl Acad Sci U S A 103:5442–5447. doi: 10.1073/pnas.0600999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava AK, Rai AN, Neilan BA. 2013. Stress biology of cyanobacteria: molecular mechanisms to cellular responses. CRC Press, Boca Raton, FL. [Google Scholar]
- 4.Li Z, Yuan S, Jia H, Gao F, Zhou M, Yuan N, Wu P, Hu Q, Sun D, Luo H. 2017. Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol J 15:433–446. doi: 10.1111/pbi.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tognetti VB, Palatnik JF, Fillat MF, Melzer M, Hajirezaei M-R, Valle EM, Carrillo N. 2006. Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 18:2035–2050. doi: 10.1105/tpc.106.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savakis P, Hellingwerf KJ. 2015. Engineering cyanobacteria for direct biofuel production from CO2. Curr Opin Biotechnol 33:8–14. doi: 10.1016/j.copbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Liberton M, Cliften PF, Head RD, Jacobs JM, Smith RD, Koppenaal DW, Brand JJ, Pakrasi HB. 2015. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci Rep 5:8132. doi: 10.1038/srep08132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungerer J, Lin PC, Chen HY, Pakrasi HB. 2018. Adjustments to photosystem stoichiometry and electron transfer proteins are key to the remarkably fast growth of the cyanobacterium Synechococcus elongatus UTEX 2973. mBio 9:e02327-17. doi: 10.1128/mBio.02327-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schottler MA, Toth SZ, Boulouis A, Kahlau S. 2015. Photosynthetic complex stoichiometry dynamics in higher plants: biogenesis, function, and turnover of ATP synthase and the cytochrome b6f complex. J Exp Bot 66:2373–2400. doi: 10.1093/jxb/eru495. [DOI] [PubMed] [Google Scholar]
- 10.Allakhverdiev SI, Nishiyama Y, Takahashi S, Miyairi S, Suzuki I, Murata N. 2005. Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol 137:263–273. doi: 10.1104/pp.104.054478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell MD, Koop R, Vasil'ev S, Bruce D. 2002. Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria. A structure-based model for the light state transition. Plant Physiol 130:1201–1212. doi: 10.1104/pp.009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fork DC, Herbert SK. 1993. Electron transport and photophosphorylation by Photosystem I in vivo in plants and cyanobacteria. Photosynth Res 36:149–168. doi: 10.1007/BF00033035. [DOI] [PubMed] [Google Scholar]
- 13.Kirilovsky D, Kerfeld CA. 2012. The orange carotenoid protein in photoprotection of Photosystem II in cyanobacteria. Biochim Biophys Acta 1817:158–166. doi: 10.1016/j.bbabio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee M, Raghavan PS, Ballal A, Rajaram H, Apte SK. 2013. Oxidative stress management in the filamentous, heterocystous, diazotrophic cyanobacterium, Anabaena PCC7120. Photosynth Res 118:59–70. doi: 10.1007/s11120-013-9929-8. [DOI] [PubMed] [Google Scholar]
- 15.Nouri MZ, Moumeni A, Komatsu S. 2015. Abiotic stresses: insight into gene regulation and protein expression in photosynthetic pathways of plants. Int J Mol Sci 16:20392–20416. doi: 10.3390/ijms160920392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe R, Iino R, Shimabukuro K, Yoshida M, Noji H. 2008. Temperature-sensitive reaction intermediate of F1-ATPase. EMBO Rep 9:84–90. doi: 10.1038/sj.embor.7401135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson A, Boulay C, Wilde A, Kerfeld CA, Kirilovsky D. 2007. Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins. Plant Cell 19:656–672. doi: 10.1105/tpc.106.045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni RD, Golden SS. 1994. Adaptation to high light intensity in Synechococcus sp. strain PCC 7942: regulation of three psbA genes and two forms of the D1 protein. J Bacteriol 176:959–965. doi: 10.1128/jb.176.4.959-965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Díaz-Troya S, López-Maury L, Sánchez-Riego AM, Roldán M, Florencio FJ. 2014. Redox regulation of glycogen biosynthesis in the cyanobacterium Synechocystis sp. PCC 6803: analysis of the AGP and glycogen synthases. Mol Plant 7:87–100. doi: 10.1093/mp/sst137. [DOI] [PubMed] [Google Scholar]
- 20.Song K, Tan X, Liang Y, Lu X. 2016. The potential of Synechococcus elongatus UTEX 2973 for sugar feedstock production. Appl Microbiol Biotechnol 100:7865–7875. doi: 10.1007/s00253-016-7510-z. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa A, Kramer DM. 2002. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci U S A 99:12789–12794. doi: 10.1073/pnas.182427499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schottler MA. 2011. ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23:304–321. doi: 10.1105/tpc.110.079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Liu S, Takano T. 2008. Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana. Biotechnol Lett 30:1289–1294. doi: 10.1007/s10529-008-9685-6. [DOI] [PubMed] [Google Scholar]
- 24.Norio M, Yoshitaka N. 2018. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ 41:285–299. doi: 10.1111/pce.13108. [DOI] [PubMed] [Google Scholar]
- 25.Allakhverdiev SI, Murata N. 2004. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32. doi: 10.1016/j.bbabio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. 2007. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421. doi: 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Soitamo AJ, Zhou G, Clarke AK, Öquist G, Gustafsson P, Aro EM. 1996. Over-production of the D1:2 protein makes Synechococcus cells more tolerant to photoinhibition of photosystem II. Plant Mol Biol 30:467–478. doi: 10.1007/BF00049325. [DOI] [PubMed] [Google Scholar]
- 28.Osman C, Wilmes C, Tatsuta T, Langer T. 2007. Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol Biol Cell 18:627–635. doi: 10.1091/mbc.e06-09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackerman SH, Tzagoloff A. 1990. Identification of two nuclear genes (ATP11, ATP12) required for assembly of the yeast F1-ATPase. Proc Natl Acad Sci U S A 87:4986–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefebvre-Legendre L, Vaillier J, Benabdelhak H, Velours J, Slonimski PP, di Rago JP. 2001. Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F1-ATPase in heat stress conditions. J Biol Chem 276:6789–6796. doi: 10.1074/jbc.M009557200. [DOI] [PubMed] [Google Scholar]
- 31.Papathanassiu AE, MacDonald NJ, Bencsura A, Vu HA. 2006. F1Fo-ATP synthase functions as a co-chaperone of Hsp90-substrate protein complexes. Biochem Biophys Res Commun 345:419–429. doi: 10.1016/j.bbrc.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 32.Trautmann D, Voß B, Wilde A, Al-Babili S, Hess WR. 2012. Microevolution in cyanobacteria: re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res 19:435–448. doi: 10.1093/dnares/dss024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter RD. 1986. Transformation in cyanobacteria. Crit Rev Microbiol 13:111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- 34.Grigorieva G, Shestakov S. 1982. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol Lett 13:367–370. doi: 10.1111/j.1574-6968.1982.tb08289.x. [DOI] [Google Scholar]
- 35.Golden SS, Sherman LA. 1984. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol 158:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elhai J, Wolk CP. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol 167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 37.Griese M, Lange C, Soppa J. 2011. Ploidy in cyanobacteria. FEMS Microbiol Lett 323:124–131. doi: 10.1111/j.1574-6968.2011.02368.x. [DOI] [PubMed] [Google Scholar]
- 38.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. [Google Scholar]
- 39.Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X. 2011. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab Eng 13:169–176. doi: 10.1016/j.ymben.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxf) 41:95–98. [Google Scholar]
- 41.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai GZ, Qiu BS, Forchhammer K. 2014. Ammonium tolerance in the cyanobacterium Synechocystis sp. strain PCC 6803 and the role of the psbA multigene family. Plant Cell Environ 37:840–851. doi: 10.1111/pce.12202. [DOI] [PubMed] [Google Scholar]
- 46.Bakels RH, van Walraven HS, Krab K, Scholts MJ, Kraayenhof R. 1993. On the activation mechanism of the H+-ATP synthase and unusual thermodynamic properties in the alkalophilic cyanobacterium Spirulina platensis. Eur J Biochem 213:957–964. doi: 10.1111/j.1432-1033.1993.tb17840.x. [DOI] [PubMed] [Google Scholar]
- 47.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 48.Ralph P, Gademann R, Larkum A, Kühl M. 2002. Spatial heterogeneity in active chlorophyll fluorescence and PSII activity of coral tissues. Mar Biol 141:639–646. doi: 10.1007/s00227-002-0866-x. [DOI] [Google Scholar]
- 49.Genty B, Briantais J-M, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 50.Jiang HB, Lou WJ, Du HY, Price NM, Qiu BS. 2012. Sll1263, a unique cation diffusion facilitator protein that promotes iron uptake in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol 53:1404–1417. doi: 10.1093/pcp/pcs086. [DOI] [PubMed] [Google Scholar]
- 51.Volkmer T, Schneider D, Bernat G, Kirchhoff H, Wenk SO, Rogner M. 2007. Ssr2998 of Synechocystis sp. PCC 6803 is involved in regulation of cyanobacterial electron transport and associated with the cytochrome b6f complex. J Biol Chem 282:3730–3737. doi: 10.1074/jbc.M604948200. [DOI] [PubMed] [Google Scholar]
- 52.Salomon E, Bar-Eyal L, Sharon S, Keren N. 2013. Balancing photosynthetic electron flow is critical for cyanobacterial acclimation to nitrogen limitation. Biochim Biophys Acta 1827:340–347. doi: 10.1016/j.bbabio.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 53.Jiang H-B, Lou W-J, Ke W-T, Song W-Y, Price NM, Qiu B-S. 2015. New insights into iron acquisition by cyanobacteria: an essential role for ExbB-ExbD complex in inorganic iron uptake. ISME J 9:297–309. doi: 10.1038/ismej.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mo H, Xie X, Zhu T, Lu X. 2017. Effects of global transcription factor NtcA on photosynthetic production of ethylene in recombinant Synechocystis sp. PCC 6803. Biotechnol Biofuels 10:145. doi: 10.1186/s13068-017-0832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.