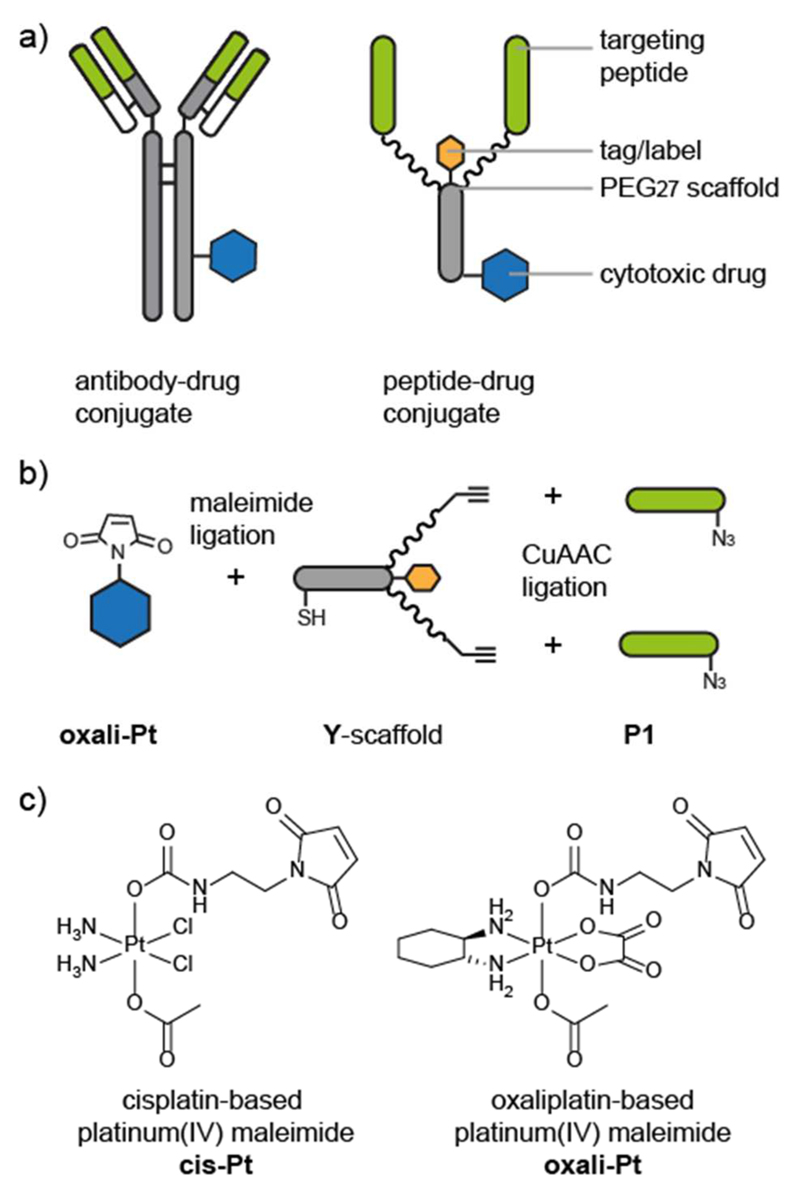
Figure 1. Design and assembly of peptide−drug conjugates.
(a) Comparison between a schematic antibody−drug conjugate and a schematic peptide−drug conjugate. The antigen-binding regions (green bar) of the antibody bind to specific receptors on cancer cells and are used to target the cytotoxic drug (blue hexagon) to the cancer cells. The peptide−drug conjugate is composed of targeting peptides (green) that bind to cancer cell receptors and a polyethylene glycol (PEG)−peptide scaffold that is conjugated to the cytotoxic drug. Chemical synthesis allows the inclusion of a label or tag (orange hexagon) and precise control over the targeting-moiety-to-drug ratio and location. (b) Modular synthesis and conjugation of the peptide−drug conjugate components. The maleimide-functionalized cytotoxic drug (oxali-Pt) is conjugated to a thiol on the peptide−PEG scaffold (Y-scaffold). The targeting peptides (P1) are conjugated to the scaffold via a copper-catalyzed azide−alkyne click (CuAAC) ligation. (c) Structures of maleimide-functionalized cis- and oxaliplatin-based platinum(IV) complexes cis-Pt and oxali-Pt.17