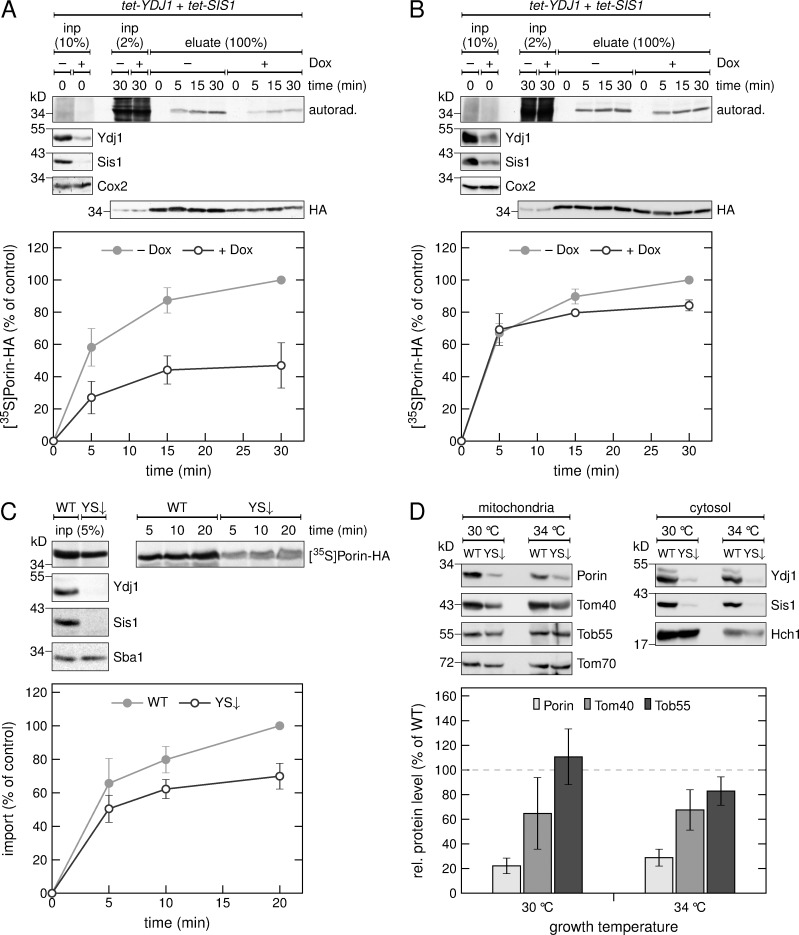
Figure 5.
The cochaperones Ydj1 and Sis1 are required for the in vivo biogenesis of Porin. (A and B) HA-tagged Porin was expressed in a strain with tetracycline-repressible promoters controlling the expression of YDJ1 and SIS1 in the absence (−) or presence (+) of doxycycline (Dox) followed by methionine starvation. Synthesis of radiolabeled proteins was initiated by addition of [35S]Met to the medium, and cells were harvested after the indicated time periods. Then, a crude mitochondrial fraction (A) or the whole cell lysate (B) were obtained. The samples were solubilized and subjected to a pull-down with anti-HA beads. Input samples from the whole cell lysate (inp) and the eluates were analyzed by SDS-PAGE, autoradiography (autorad.) and immunodecoration with the indicated antibodies. Bottom, intensities of the bands corresponding to Porin-HA from three independent experiments were quantified, and the mean intensity from the 30-min samples without doxycycline was set to 100%. Error bars represent ± SD. (C) Top, radiolabeled Porin-HA was translated in yeast extract from WT yeast cells or from cells depleted for Ydj1 and Sis1 (YS↓). The translation reactions were subjected to in vitro import reactions using mitochondria isolated from YS↓ cells. After import for the indicated times, the mitochondria were subjected to carbonate extraction. The samples were analyzed by SDS-PAGE, autoradiography ([35S]Porin-HA) and immunodecoration with the indicated antibodies. Bottom, the intensities of the bands corresponding to Porin-HA were quantified and the intensity from import of Porin-HA translated in the WT strain was set to 100%. Error bars represent ± SD. (D) Top, a crude mitochondrial and a cytosolic fraction were isolated from WT or YS↓ yeast cells analyzed by SDS-PAGE and immunodecoration with the indicated antibodies. Bottom, intensities of the bands in the mitochondrial fraction from three independent experiments were quantified and normalized to the level of Tom70. The level of the proteins in the WT strain was set to 100%. Error bars represent ± SD.