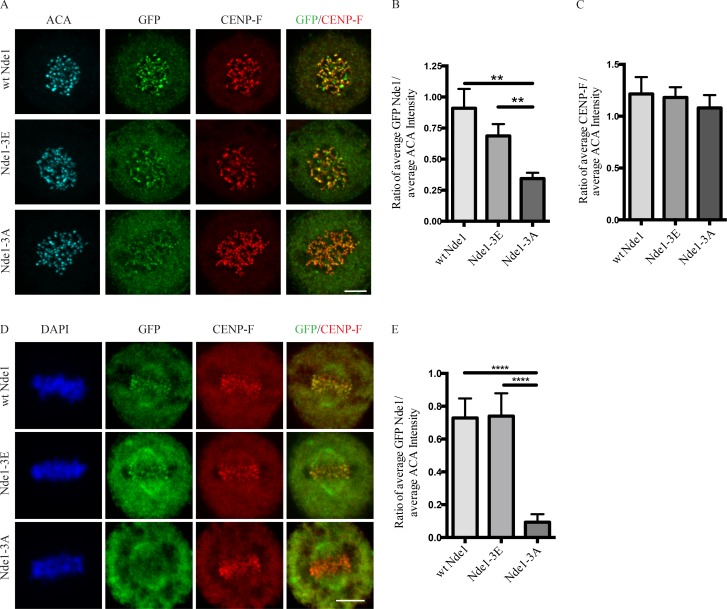
Figure 2.
Effects of Nde1 phosphorylation on localization at unattached kinetochores. (A) Distribution of GFP-WT and phosphorylation-state mutant Nde1 in nocodazole-treated HeLa cells showing clear localization of each construct to kinetochores in the absence of MTs. The intensity of phosphomutant Nde1 appeared to be decreased relative to WT and phosphomimetic nde1. Bar, 5 µm. (B) Quantification of (anti-GFP vs. anti-ACA immunofluorescence intensity). Student’s t test showed a significant decrease in phosphomutant Nde1 intensity relative to the other conditions. **, P < 0.005. (C) Quantification of mean CENP-F intensity relative to mean ACA immunofluorescence signal. ANOVA statistical analysis showed no significant difference among the three conditions. Mean ± SEM for three independent experiments is represented. (D) HeLa cells transfected Nde1 phosphorylation-state cDNAs were blocked at metaphase with the proteasome inhibitor MG132, and kinetochores were examined for GFP immunofluorescence intensity. The phosphomutant Nde1-3A was absent from the kinetochores, revealing that Cdk1 phosphorylation of Nde1 blocks its dynein-mediated removal from these sites. Bar, 5 μm. (E) Quantification of GFP Nde1 signal intensity at kinetochores relative to ACA immunofluorescence. Student’s t test identified a significant decrease in levels of GFP Nde1-3A at kinetochores. ****, P < 0.0001.