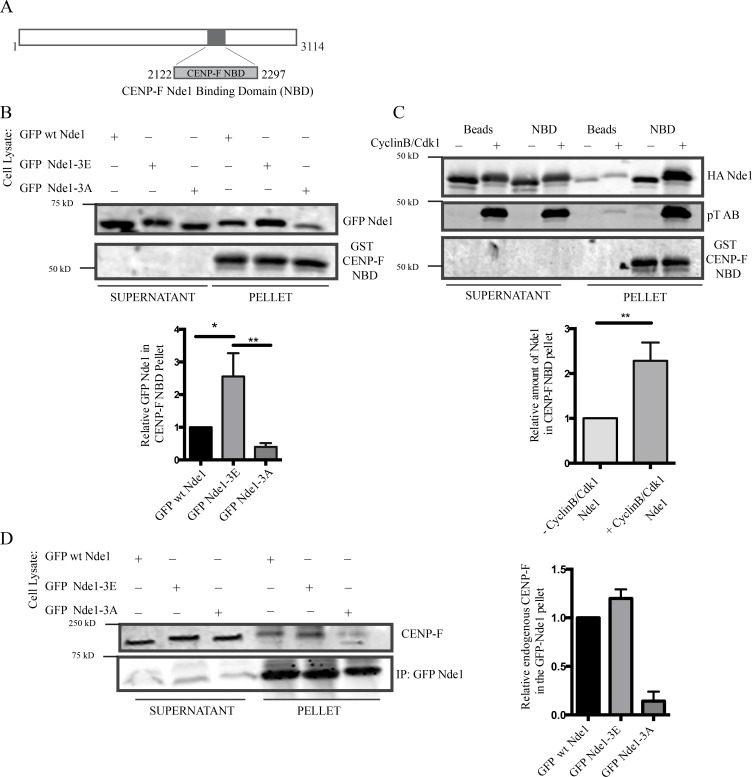
Figure 3.
Nde1 Cdk1 phosphorylation of Nde1 enhances CENP-F interaction. (A) Diagram showing CENP-F fragment used for biochemical analysis (NBD: amino acids 2,122–2,297). (B) GST-CENP-F NBD pull-down of GPF-Nde1 constructs from HeLa cells demonstrates increased binding of GFP-phosphomimetic Nde1. (C) GST-CENP-F NBD pull-down of bacterially expressed HA-Nde1 after in vitro phosphorylation with recombinant Cdk1/CyclinB. Levels of Nde1 in the CENP-F NBD pull-downs were quantified and again revealed an increased binding of Cdk1-phosphorylated Nde1 to the GST CENP-F NBD fragment. (D) Anti-GFP immunoprecipitation of endogenous CENP-F with GFP-tagged Nde1 phosphorylation state constructs. Immunoprecipitation of GFP WT Nde1 and GFP-Nde1-3E each specially coprecipitated endogenous CENP-F. Mean ± SEM of three independent experiments is represented. **, P < 0.005; *, P < 0.05.