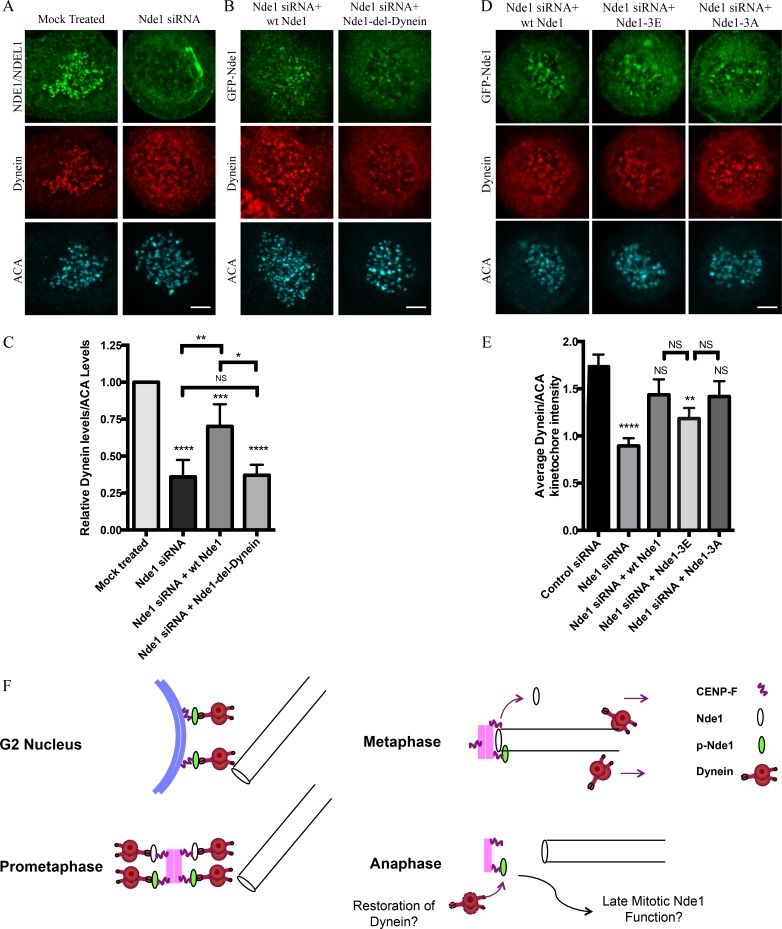
Figure 5.
Requirement of Nde1 for dynein recruitment to the kinetochore. (A) HeLa cells treated with Nde1 siRNA, exposed to nococazole, and immunostained for endogenous NDE1/NDEL1 or dynein intermediate chain. (B) Nde1 siRNA-treated HeLa cells rescued with expression of GFP WT Nde1 or GFP Nde1-del-dynein and stained for GFP and dynein intermediate chain. (C) Quantification of mean kinetochore dynein levels relative to mean ACA immunofluorescence signal. Dynein/ACA values were plotted ± SEM. Paired Student’s t test was performed to analyze significance between conditions. (D) Nde1 siRNA-treated HeLa cells rescued by expression of WT Nde1, Nde1-3E, or Nde1-3A and stained for GFP and dynein intermediate chain. Bars, 5 µm. (E) Quantification of mean dynein intensity relative to mean ACA immunofluorescence signal. Mean ± SEM of three independent experiments is represented. Student’s t tests were performed to analyze significance between conditions and revealed a significant difference in the levels of kinetochore dynein between Nde1 siRNA and WT Nde1, Nde1-3E, or Nde1-3A. *, P < 0.05; **, P < 0.005; ***, P < 0.001; ****, P < 0.0001. (F) Role of Cdk1 phosphorylation in G2-M Nde1 behavior. Phosphorylated Nde1 is shown associating with the G2 NE and then kinetochores from prophase into anaphase when dephosphorylated Nde1 is lost from these sites. Although dynein is undetectable at normal anaphase kinetochores, we speculate that persistent pNde1 might serve in a late dynein-mediated kinetochore attachment correction mechanism.