Abstract
Neuroendocrine tumors, or carcinoid tumors, of both the midgut and hindgut are quite rare, but their incidence is increasing. Surgery is the treatment of choice in patients who can tolerate an operation and have operable disease. Options for the treatment of metastatic disease include cytoreductive surgery, somatostatin analogues, interferon α, local liver therapies (hepatic arterial embolization, ablation), chemotherapy, Peptide-Receptor Radionucleotide Radiotherapy, angiogenesis inhibitors, and mammalian target of rapamycin inhibitors.
Keywords: neuroendocrine tumors, carcinoid tumors, jejunoileal NET, appendiceal NET, colorectal NET
A neuroendocrine tumor (NET) of the gastrointestinal (GI) tract was first described by Langhans in 1867. Forty years later in 1907, Oberndorfer coined the term karzinoide to refer to these lesions, and to distinguish what he thought were benign tumors from malignant GI tumors, such as adenocarcinoma. 1 These neoplasms have been widely referred to as carcinoids since, recently, however, the term “NET” has become preferable, and is used by the two main NET societies, the North American Neuroendocrine Tumor Society (NANETS) and the European Neuroendocrine Tumor Society (ENETS). This article will focus on NETs of the midgut (jejunum, ileum, appendix, and cecum), as well as the hindgut (distal colon and rectum).
Epidemiology
Midgut
NETs of both the midgut and hindgut are quite rare. Data from the Surveillance, Epidemiology, and End Results (SEER) program database estimate an incidence of 0.67 per 100,000 per year for NETs of the jejunum and ileum, 0.16 per 100,000 annually for appendiceal NETs, and 0.15 per 100,000 annually for cecal NETs. 2 Males and females are affected equally for appendiceal and cecal NETs; males, however, are affected more often by NETs of the jejunum and ileum, with an M:F ratio of ∼1.4:1. 2 There are racial disparities in the incidence of midgut NETs, with those of black race more likely to be diagnosed with NETs of the jejunum, ileum, and cecum than their white and Asian counterparts. For appendiceal NETs, whites are more likely to be affected than those of black race, who are in turn more likely to be affected than Asians. 2 The consumption of high levels of saturated fats has been shown to be a statistically significant risk factor for the development of midgut NETs, 3 while high-fiber diets are thought to be protective against the disease. 4 No association has been shown for alcohol consumption or tobacco use.
Hindgut
NETs of the colon and rectum remain quite rare, but their incidence is increasing. 5 SEER data demonstrate an increase in the incidence of colonic NETs in the United States, from 0.02 per 100,000 in 1973 to 0.2 per 100,000 in 2004. European data have indicated a lower incidence of 0.06 per 100,000. 6 Rectal NETs have shown a comparable increase in incidence from 0.2 per 100,000 in 1973 to 0.86 per 100,000 in 2004, according to SEER data. Rectal NETs have a racial predominance for those of black and Asian race, with those of black race being 2.3 times more likely to have the disease than their white counterparts, and those of Asian race being 4.9 times more likely to have the disease than non-Asians. 1 2 Colonic NETs account for approximately 7.5% of all NETs in the United States, and data from Europe and Asia indicate a similar percentage. 6 7 8 9 10 Rectal NETs account for 18% of all NETs in the United States and 27% of all GI NETs. 2 The numbers are lower in Europe, where rectal NETs account for 5 to 14% of the total. 6 8 9 10 In Asia, however, rectal NETs are more prevalent, and make up 60 to 89% of all NETs of the GI tract in Japan. 10
Presentation
Midgut
The median age at diagnosis for all midgut NETs is 64 years; appendiceal NETs, however, are diagnosed at a significantly younger age than jejunoileal and cecal NETs, with a median of 47 years at diagnosis. 2 Midgut NETs are usually quite small and can remain so indefinitely. They often cause vague GI symptoms, and delay in diagnosis is very common. One study of 115 patients with abdominal NETs found that only 4% of patients received a prompt diagnosis of NET, while the other 96% of patients had a mean delay in diagnosis of 62 months, with a range from 2 months to more than 20 years. 11 In that study, the most common incorrect diagnosis was irritable bowel syndrome (IBS), with about half of the patients diagnosed with IBS. 11 A majority of appendiceal NETs are diagnosed on pathology after appendectomy for suspected appendicitis. Symptoms caused by jejunoileal and cecal NETs are related either to obstruction from the tumor itself, mesenteric nodal metastases, or from bowel ischemia due to mesenteric involvement of the tumor. These tumors commonly metastasize to lymph nodes at the root of the mesentery near the main trunks of the superior mesenteric vein and artery. These nodal metastases can become quite large, and often cause a significant desmoplastic reaction that shortens the mesentery and causes multiple hairpin turns in the bowel. These mesenteric masses are often the cause of obstruction and ischemia in these patients. It is not uncommon for patients to have multiple episodes of small bowel obstruction that resolve with nonoperative management.
At the time of diagnosis, 60 to 80% of patients with small bowel NETs already have liver metastases. 12 Because of this, patients with midgut NETs can present with symptoms of carcinoid syndrome: diarrhea, flushing, right-sided heart failure due to valvular disease, and bronchoconstriction. This syndrome is almost exclusively seen with metastatic disease. Of patients who present with the carcinoid syndrome, 95% have liver metastases. 13
Hindgut
The mean age at diagnosis is 65 years for colonic NETs and 56 years for rectal NETs. 14 Approximately 50% of patients are asymptomatic at the time of diagnosis, and the tumor is usually found incidentally on endoscopy. The carcinoid syndrome is exceedingly rare in patients with colorectal NETs. Colonic NETs tend to present later with larger tumors and more frequently have distant metastases at the time of diagnosis, with only 45% of colonic NETs localized at diagnosis. 14
Sites of metastasis include liver, lymph nodes, mesentery, and peritoneum. When patients have symptoms, they include diarrhea, abdominal pain, GI bleed, and weight loss. Obstruction is also possible; it is, however, quite rare. Rectal NETs tend to present as a localized tumor (75–85%), with few patients presenting with distant metastases (2–8%). 15 Sites of metastasis include bone, liver, and lymph nodes. When symptoms are present, the most common is bright red blood per rectum. Rectal NETs can also, but rarely, cause anorectal symptoms such as pain and tenesmus. They are found at a median of 8 cm from the anal verge, and endoscopically appear as small (median size of 6 mm), polypoid, subepithelial lesions that often appear to be yellow in color due to the presence of chromogranin A (CgA), and are sometimes mistaken for lipomas. 16 17
Pathology/Prognosis
NANETS has published a “minimum pathology dataset” that details the information that should be included on a surgical pathology report for NETs. This dataset applies to both midgut and hindgut NETs and includes site of tumor, diagnosis, three-dimensional size, presence of unusual histology, presence of multicentric disease, staining for chromogranin and synaptophysin, grade, mitotic rate or Ki-67 index, presence of tumor necrosis, extent and depth of invasion, involvement or invasion of serosal and peritoneal surfaces and adjacent structures, presence of vascular invasion, presence of perineural invasion, lymph node metastases (number of positive nodes/number of nodes examined), TNM staging, and resection margins. 18 The grading system for NETs divides tumors into three categories (low, intermediate, and high grades). The grades are mainly separated, based on proliferative rate. This can be measured either by using mitotic rate and counting 50 high-power fields (HPFs), or by using Ki-67 labeling.
Midgut
TNM staging systems exist for NETs, based on the anatomic site of the tumor. The TNM classifications for jejunoileal NETs are shown in Tables 1 and 2 . T1 tumors are defined as those less than 1 cm in size that do not invade the muscularis propria. Tumors invading the muscularis propria and all tumors >1 cm are classified as T2. Tumors invading the subserosa are T3, and those invading the peritoneum or other organs are T4. 19 The TNM classification for appendiceal NETs is different and is shown in Tables 3 and 4 . T1 tumors are defined as those less than 2 cm, and not invading the subserosa or mesoappendix. Tumors that are between 2 and 4 cm without subserosal or mesoappendix invasion are classified as T2. Tumors more than 4 cm and all tumors with subserosal or mesoappendix invasion are T3, and those invading other organs are T4. 20
Table 1. TNM classification for jejunoileal NETs 19 .
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Invades lamina propria or submucosa and ≤ 1 cm in size |
| T2 | Invades muscularis propria or ≥ 1 cm in size |
| T3 | Invades through the muscularis propria into subserosal tissue without penetration of overlying serosa |
| T4 | Invades visceral peritoneum or other organs or adjacent structures |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis < 12 nodes |
| N2 | Large mesenteric masses (>2 cm) and/or extensive nodal deposits (≥ 12 nodes), especially those that encase the superior mesenteric vessels |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confirmed to the liver |
| M1b | Metastases in at least one extrahepatic site |
| M1c | Both hepatic and extrahepatic metastases |
Abbreviation: NETs, neuroendocrine tumors.
Table 2. Prognostic stage groups for jejunoileal NETs 19 .
| T | N | M | Stage |
|---|---|---|---|
| T1 | N0 | M0 | I |
| T1 | N1, N2 | M0 | III |
| T1 | N0, N1, N2 | M1 | IV |
| T2 | N0 | M0 | II |
| T2 | N1, N2 | M0 | III |
| T2 | N0, N1, N2 | M1 | IV |
| T3 | N0 | M0 | II |
| T3 | N1, N2 | M0 | III |
| T3 | N0, N1, N2 | M1 | IV |
| T4 | N0 | M0 | III |
| T4 | N1, N2 | M0 | III |
| T4 | N0, N1, N2 | M1 | IV |
Abbreviation: NETs, neuroendocrine tumors.
Table 3. TNM classification for appendiceal NETs 20 .
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | ≤ 2 cm in greatest dimension |
| T2 | Between 2 and 4 cm in greatest dimension |
| T3 | > 4 cm or with subserosal invasion or involvement of the mesoappendix |
| T4 | Tumor perforated the peritoneum or directly invades other adjacent organs or structures (excluding direct mural extension to adjacent subserosa of adjacent bowel), e.g., abdominal wall and skeletal muscle |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confirmed to the liver |
| M1b | Metastases in at least one extrahepatic site |
| M1c | Both hepatic and extrahepatic metastases |
Abbreviation: NETs, neuroendocrine tumors.
Table 4. Prognostic stage groups for appendiceal NETs 20 .
| T | N | M | Stage |
|---|---|---|---|
| T1 | N0 | M0 | I |
| T1 | N1 | M0 | III |
| T1 | N0, N1 | M1 | IV |
| T2 | N0 | M0 | II |
| T2 | N1 | M0 | III |
| T2 | N0, N1 | M1 | IV |
| T3 | N0 | M0 | II |
| T3 | N1 | M0 | III |
| T3 | N0, N1 | M1 | IV |
| T4 | N0 | M0 | III |
| T4 | N1 | M0 | III |
| T4 | N0, N1 | M1 | IV |
Abbreviation: NETs, neuroendocrine tumors.
Five-year survival for midgut NETs varies significantly, based on anatomic site, with appendiceal NETs having the most favorable survival at 88% with localized disease. Five-year survival in localized disease for jejunoileal and cecal NETs is 65 and 68%, respectively. The presence of regional lymph node metastasis does not significantly affect prognosis for jejunoileal and cecal disease, with 5-year survival rates of 71% for both jejunoileal and cecal NETs with regional disease. Lymph node metastasis does confer a poorer prognosis on patients with appendiceal NET, with 5-year survival 78% for NETs of the appendix with regional disease. 18
Prognosis for appendiceal NETs is highly dependent on the size of the tumor, the presence of lymphovascular invasion (LVI) and mesoappendix invasion, and the location of the tumor on the appendix. A majority of appendiceal NETs are confined to the tip of the appendix, with only 10% of tumors involving the appendiceal base. Appendiceal NETs ≤ 1 cm in size have a 5-year survival of 95%, while the 5-year survival for patients with tumors ≥ 2 cm is 70.5%. 18 Prognosis for jejunoileal NETs also depends on size. Tumors >1.5 cm will usually have metastatic disease at the time of diagnosis. 18 Grade is also an important prognostic factor, with significantly poorer survival for higher grade NETs. 13
Hindgut
Colorectal NETs also have their own TNM classification system ( Tables 5 and 6 ). T1 tumors are confined to the submucosa and are <2 cm in size. T1a refers to lesions <1 cm, while T1b is for lesions between 1 and 2 cm. T2 tumors either invade the muscularis propria, or are >2 cm, or both. T3 and T4 are identical to the T-staging for colorectal adenocarcinoma, with a T3 lesion involving the pericolorectal tissues, and a T4 lesion penetrating the visceral peritoneum. 21
Table 5. TNM classification for colorectal NETs 21 .
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Invades the lamina propria or submucosa and is ≤ 2 cm |
| T1a | < 1 cm in greatest dimension |
| T1b | 1–2 cm in greatest dimension |
| T2 | Invades the muscularis propria or is > 2 cm with invasion of the lamina propria or submucosa |
| T3 | Invades through the muscularis propria into subserosal tissue without penetration of overlying serosa |
| T4 | Invades the visceral peritoneum or other organs or adjacent structures |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Metastasis confirmed to the liver |
| M1b | Metastases in at least one extrahepatic site |
| M1c | Both hepatic and extrahepatic metastases |
Abbreviation: NETs, neuroendocrine tumors.
Table 6. Prognostic stage groups for colorectal NETs 21 .
| T | N | M | Stage |
|---|---|---|---|
| T1 | N0 | M0 | I |
| T1 | N1 | M0 | IIIB |
| T1 | N0, N1 | M1 | IV |
| T2 | N0 | M0 | IIA |
| T2 | N1 | M0 | IIIB |
| T2 | N0, N1 | M1 | IV |
| T3 | N0 | M0 | IIB |
| T3 | N1 | M0 | IIIB |
| T3 | N0, N1 | M1 | IV |
| T4 | N0 | M0 | IIIA |
| T4 | N1 | M0 | IIIB |
| T4 | N0, N1 | M1 | IV |
Abbreviation: NETs, neuroendocrine tumors.
Rectal NETs have a favorable prognosis, with a 5-year survival of 88%. 14 Colonic NETs have the worst 5-year overall survival rate of all GI NETs at 62%. 15 For colonic NETs, the 5-year survival is 76% for localized disease, 72% when there is lymph node involvement, and 32% for distant metastatic disease. The 5-year survival for rectal NETs with distant metastases is also quite poor at 30%; patients with rectal NETs, however, are much less likely to present with metastatic disease. 14
Size is a very important prognostic factor for rectal carcinoids. The risk of metastasis with tumors <1 cm is 2%. The risk for tumors 1 to 2 cm is 10 to 15%, and that for tumors >2 cm is 60 to 80%. 22 The depth of invasion is also an important factor. One study showed that for rectal tumors <2 cm, the risk of metastasis is only 2%, if there is no invasion of the muscularis propria, whereas the risk of metastasis climbs to 48% when invasion of the muscularis propria is present. 23
Fahy et al performed a retrospective analysis of 70 rectal NETs and analyzed the prognostic effects of several tumor factors. They found that patients with metastases were more likely to have tumors that were larger and deeper with LVI, and had a higher mitotic rate. Increased size of tumor was also associated with invasion into the muscularis, LVI, and increased mitotic rate. 24 Significant factors associated with decreased 5-year recurrence-free survival (RFS) were larger tumor size, muscularis invasion, and >2 mitoses per 50 HPF. These same factors were all associated with decreased 5-year disease-specific survival (DSS), with the addition of LVI. They found that 5-year RFS was 92% in tumors <1 cm, and 30% in tumors >2 cm. RFS was drastically affected by muscularis invasion, with a five-year RFS rate of 30% with muscularis invasion and 88% without muscularis invasion. LVI was associated with a 30% reduction in both RFS and DDS. 24 Based on their findings, the authors created a carcinoid of the rectum risk stratification score ( Fig. 1 ). Zero points were assigned for tumor <1 cm, no muscularis invasion, absence of LVI, and mitoses <2/50 HPF. One point was assigned for tumor 1 to 2 cm, muscularis invasion, the presence of LVI, and mitoses >2/50 HPF. Tumor size >2 cm received two points. Patients were then stratified into low risk (zero points), intermediate risk (1–2 points), and high-risk (three or more points). RFS was significantly lower in the low-risk group, when compared with both the intermediate and high-risk groups ( Fig. 2 ).
Fig. 1.
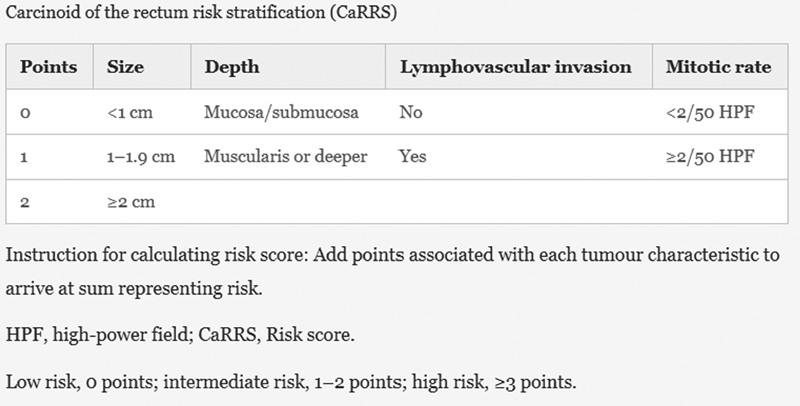
Carcinoid of the rectum risk stratification (CaRRS) scoring system.
Fig. 2.
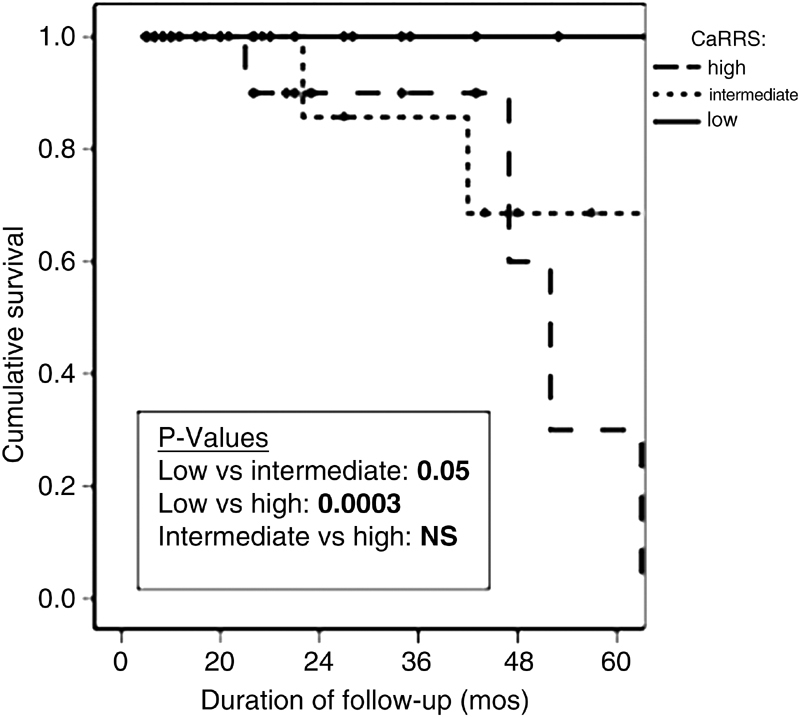
Recurrence-free survival for patients based on the carcinoid of the rectum risk stratification (CaRRS) score.
Preoperative Testing
Midgut
Initial imaging work-up should consist of a chest X-ray, as well as cross-sectional imaging of the abdomen and pelvis (computed tomography [CT] or magnetic resonance imaging [MRI]), and octreotide scintigraphy to determine the full extent of disease and evaluate for liver metastases. As the primary midgut NET is usually quite small, it is often missed on cross-sectional imaging. Instead, what are often seen are the large mesenteric nodal metastases and their associated desmoplastic reaction. Imaging should be performed with arterial and delayed contrast phases as NETs enhance during the arterial phase, and wash out on delayed phases. Octreotide scintigraphy uses a somatostatin analog radiolabeled with indium-111 ( 111 In) to visualize uptake of somatostatin throughout the body. It has a sensitivity of 80 to 90% for identifying NETs more than 1 cm. 18 Imaging is usually performed at multiple time intervals over 24 hours. Imaging at shorter time intervals demonstrates lower bowel activity, but higher background uptake, while imaging at longer intervals decreases the background uptake, but has issues with higher bowel activity. Octreotide scintigraphy is also useful to determine the status of somatostatin receptors 2 and 5 of a known NET, which can be useful to guide medical therapy for the treatment of metastatic disease. Fluorodeoxyglucose (FDG)-positron emission tomography scans are generally not used for the evaluation of NETs, as well-differentiated NETs tend to have low FDG avidity.
Laboratory testing may consist of serum CgA and urine 5′-hydroxyindoleacetic acid (5′-HIAA). One study showed the sensitivity of CgA in detecting NETs to be 92%, with a specificity of 67%. 25 Proton pump inhibitors (PPIs) also elevate CgA levels; therefore, PPI therapy must be stopped for 2 to 4 weeks prior to CgA testing to limit false-positive results. Urine 5′-HIAA testing is performed with a 24-hour collection. The specificity is 88%. 26 Foods rich in serotonin, such as bananas, pineapples, kiwis, plums, tomatoes, bell peppers, chocolates, and walnuts, as well as medications increasing serotonin levels, such as various muscle relaxants and antiemetics, should be avoided for at least 24 hours prior to urine collection. Patients exhibiting symptoms of the carcinoid syndrome, as well as those with cardiac murmurs on physical examination, should undergo echocardiography to evaluate for carcinoid heart disease.
Hindgut
A full colonoscopy should be performed, if not already done at the time of initial diagnosis to evaluate for synchronous lesions. Given the low likelihood of metastatic disease, cross-sectional imaging is not indicated for tumors <2 cm that are confined to the submucosa. For lesions >2 cm and/or with muscularis invasion, the abdomen and pelvis should be imaged with either CT or MRI to evaluate for distant metastases. To assess local invasion and lymph node involvement, pelvic MRI and/or endorectal ultrasound (ERUS) should be obtained. ERUS is excellent at gauging depth of invasion, with one study showing 100% accuracy in 52 patients. 27 Octreotide scintigraphy plays a limited role in evaluation of the primary tumor, as higher grade tumors will often not demonstrate uptake of the octreotide, and high levels of background activity make detection of a rectal primary difficult. Octreotide scans can be useful for evaluation of metastatic disease and to evaluate somatostatin receptor expression, which can play a role in medical treatments. Routine testing of serum serotonin or urine 5′-HIAA is not recommended, as colorectal NETs very rarely secrete bioactive hormones. Serum CgA is recommended, as it can be used to monitor patients with metastatic disease.
Surgical Treatment
Midgut
The surgical treatment for appendiceal NETs ranges from appendectomy to formal right hemicolectomy. Appendectomy is the appropriate operation for tumors <1 cm and is confined to the tip of the appendix. Appendiceal NETs that require right hemicolectomy include tumors >2 cm, those with LVI or invasion into the mesoappendix, high-grade NETs, tumors with positive margins after appendectomy, and those with mesenteric lymph node involvement. Tumors with unusual histology, such as adenocarcinoid and goblet cell carcinoid, tend to be more aggressive, and should be treated with a right hemicolectomy, regardless of their size. 18
The surgical treatment for cecal NETs is a right hemicolectomy. Treatment for jejunoileal NETs is surgical resection following oncologic principles. A diligent search for additional synchronous lesions, as well as metastatic disease, should be performed at the time of operation. These resections can be quite difficult and tedious, due to the tendency of these tumors to involve the mesentery and encase the mesenteric vessels, as well as the intense desmoplastic reaction that often accompanies them. Efforts should be made to preserve bowel length and the ileocecal valve when possible, as many patients will have issues with diarrhea postoperatively. 18 It is not uncommon for patients to present emergently with obstruction or ischemia. In this situation, it is reasonable to operate to relieve the life-threatening obstruction or ischemia and address the full extent of the mesenteric and liver disease, if present, at a later time. Often times, referral to an experienced center is required for involved mesenteric resections. The majority of cases of mesenteric disease that are declared “unresectable” at other hospitals are able to be successfully debulked at a center with expertise in NET surgery. 18 In patients with metastatic disease, cholecystectomy should be performed at the time of initial resection, due to the likelihood of octreotide therapy postoperatively, and its cholestatic effects. Resection of the primary tumor should be considered, even in patients with inoperable metastatic disease, as multiple studies have shown resection of the primary small bowel tumor to be an independent predictor of improved survival. 28 29
During operations for NETs, the timely recognition and treatment of carcinoid crisis are of great importance. Carcinoid crisis is characterized by hemodynamic instability and can also involve bronchospasm and flushing. Carcinoid crisis most often occurs in patients with carcinoid syndrome and those with liver metastases but can occur in any patient with NET. 18 30 Preoperative and intraoperative dosing of octreotide either by bolus or a continuous infusion is recommended; 31 however, recent studies, did not show any significant preventive effects of either method of administering octreotide. 30 32 Intraoperatively, carcinoid crises are treated with a variety of medications including octreotide, vasopressors, crystalloid boluses, histamine blockers (both histamine-1 and histamine-2), and steroids. Vasopressors appear to be the most effective treatment, with vasopressin and phenylephrine being the preferred agents. 30
Small well-differentiated appendiceal NETs treated with appendectomy do not generally require long-term surveillance. All other midgut NETs should undergo long-term surveillance for at least 7 years and often continue lifelong. 13 18 Follow-up should occur every 6 to 12 months, and consist of history and physical examination, chest X-ray, and CT or MRI of the abdomen and pelvis. Surveillance laboratory testing with serum CgA and urine 5'-HIAA can be considered on an individual basis. The role of octreotide scintigraphy for surveillance is controversial. 13 18
Hindgut
Colonic NETs, for the most part, are treated like adenocarcinoma. Tumors <2 cm may be resected endoscopically, but an oncologic resection is required if the lesion is incompletely excised, or if the tumor is high grade. As most colonic NETs are >2 cm and invade the muscularis propria, endoscopic resection alone is not appropriate for the majority of colonic NETs. An oncologic resection with colectomy and resection of the associated lymph drainage is required.
Given that rectal NETs range from small and indolent to large and aggressive, it follows that the surgical options vary from minor endoscopic polypectomy to major low anterior resection (LAR) or abdominoperineal resection with total mesorectal excision (TME). For lesions <2 cm and confined to the submucosa, local excision (endoscopic polypectomy or transanal excision) is appropriate. One study of 41 endoscopic polypectomies for rectal NETs demonstrated positive margins in seven patients (17%). Three of the seven patients underwent further resection, while the other four did not. One of the four locally recurred 16 years after treatment. 27 Local excision may also be considered in lesions <2 cm with muscularis invasion with ERUS negative for lymph node involvement. Tumors that are >2 cm, invading the muscularis, or involving lymph nodes should be treated like adenocarcinoma and undergo TME.
For lesions <2 cm that are confined to the submucosa, there is no role for long-term surveillance imaging, as the risk of metastasis is so low. For tumors with muscularis invasion or lymph node involvement, long-term surveillance imaging is warranted with yearly CT or MRI. Surveillance serum CgA levels can also be useful, but CgA levels will be elevated in patients on PPIs and those with chronic gastritis or other chronic inflammatory condition.
Treatment of Metastatic Disease
Options for the oncologic treatment of metastatic disease include cytoreductive surgery, somatostatin analogues, interferon α, local liver therapies (hepatic arterial embolization, ablation), chemotherapy, Peptide-Receptor Radiotargeted Radiotherapy (PRRT), angiogenesis inhibitors, mammalian target of rapamycin (mTOR) inhibitors. Cytoreductive surgery is the treatment of choice in patients who can tolerate an operation and have operable disease. The liver is by far the most common site of metastasis, and liver failure from metastases is the most frequent cause of death in patients with NETs. 18 Cytoreduction with a debulking threshold of ≥70% has been shown to have favorable results, with a 5-year DSS of 90%. 12
Somatostatin analogs (e.g., octreotide, lanreotide) are effective in treating symptoms related to carcinoid syndrome. They also have been shown to significantly inhibit tumor growth in two separate randomized controlled trials. The PROMID trial showed that patients with metastatic midgut NETs taking octreotide long-acting repeatable (LAR) had a significantly longer time to disease progression than those taking placebo. 33 The CLARINET trial demonstrated a similar progression-free survival prolonging effect of lanreotide among patients with metastatic NETs of midgut, hindgut, or pancreatic origin. 34 Somatostatin analogs have also been shown to have tumor inhibiting activity in a human rectal NET cell line, using a mouse model. 35 Octreotide is therefore a reasonable option for treatment of metastatic midgut and hindgut NETs, if octreotide scan confirms tumor somatostatin receptor uptake.
Systemic chemotherapy is rarely used for treatment of metastatic NETs. The regimen used for NET includes streptozotocin, 5-fluorouracil, and doxorubicin. Results with this regimen have been varied, and its side effect profile can be prohibitive. Current areas of research include antiangiogenesis agents, such as bevacizumab and sunitinib, as well as mTOR inhibitors, such as everolimus and temsirolimus. 36 Data from the RADIANT-4 trial were recently published. This was a randomized controlled trial investigating the efficacy of everolimus in patients with metastatic NETs with a GI or pulmonary primary. Everolimus was shown to significantly improve progression-free survival, with a median progression-free survival of 11.0 months in patients taking everolimus, compared with a progression-free survival of 3.9 months in patients on placebo. 37
One exciting therapy under investigation is PRRT, which involves the use of radiolabeled octreotide, and is currently only available in Europe. The two most common radioactive drugs used in therapy are 90 Y-DOTA-Phe 1 -Tyr 3 -Octreotide ( 90 Y-DOTATOC) and 177 Lu-DOTA-Tyr 3 -octreotate ( 177 Lu-DOTATATE). 18 A recent randomized controlled trial (NETTER-1), randomized patients with metastatic midgut NET to treatment with octreotide LAR or PRRT with 177 Lu-DOTATATE. The patients in the PRRT group had significantly longer progression-free survival and higher radiographic response rates than patients in the octreotide group. 38 This trial highlights the promise of PRRT as a treatment for metastatic NET going forward.
Conclusion
NETs of the small bowel, colon, and rectum are relatively rare, but their incidence is increasing. These tumors behave quite differently from other more common tumors of the GI tract. They can range from small localized tumors that can be treated definitively, with endoscopic resection to aggressive tumors, with widespread mesenteric and liver metastases, requiring extensive liver debulking and complex mesenteric dissections. The mortality associated with this disease is almost exclusively due to the extent of liver metastases, and there are multiple options available to treat metastatic disease. Recent data demonstrate the promise of new therapies for metastatic disease and highlight the importance of ongoing research in this exciting field.
References
- 1.Modlin I M, Lye K D, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(04):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Yao J C, Hassan M, Phan A et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Cross A J, Leitzmann M F, Subar A F, Thompson F E, Hollenbeck A R, Schatzkin A. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res. 2008;68(22):9274–9279. doi: 10.1158/0008-5472.CAN-08-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schatzkin A, Park Y, Leitzmann M F, Hollenbeck A R, Cross A J. Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology. 2008;135(04):1163–1167. doi: 10.1053/j.gastro.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avenel P, McKendrick A, Silapaswan S et al. Gastrointestinal carcinoids: an increasing incidence of rectal distribution. Am Surg. 2010;76(07):759–763. [PubMed] [Google Scholar]
- 6.Niederle M B, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17(04):909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 7.Jetmore A B, Ray J E, Gathright J B, Jr, McMullen K M, Hicks T C, Timmcke A E. Rectal carcinoids: the most frequent carcinoid tumor. Dis Colon Rectum. 1992;35(08):717–725. doi: 10.1007/BF02050318. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21(09):1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- 9.Ploeckinger U, Kloeppel G, Wiedenmann B, Lohmann R; representatives of 21 German NET Centers.The German NET-registry: an audit on the diagnosis and therapy of neuroendocrine tumors Neuroendocrinology 20099004349–363. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Sasano H, Tanaka M et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45(02):234–243. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 11.Toth-Fejel S, Pommier R F. Relationships among delay of diagnosis, extent of disease, and survival in patients with abdominal carcinoid tumors. Am J Surg. 2004;187(05):575–579. doi: 10.1016/j.amjsurg.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Baker A N, Sauer D A, Pommier S J, Pommier R F.Expanded criteria for carcinoid liver debulking: maintaining survival and increasing the number of eligible patients Surgery 2014156061369–1376., discussion 1376–1377 [DOI] [PubMed] [Google Scholar]
- 13.Pape U F, Perren A, Niederle B et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95(02):135–156. doi: 10.1159/000335629. [DOI] [PubMed] [Google Scholar]
- 14.Anthony L B, Strosberg J R, Klimstra D S et al. The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39(06):767–774. doi: 10.1097/MPA.0b013e3181ec1261. [DOI] [PubMed] [Google Scholar]
- 15.Caplin M, Sundin A, Nillson O et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95(02):88–97. doi: 10.1159/000335594. [DOI] [PubMed] [Google Scholar]
- 16.Shields C J, Tiret E, Winter D C; International Rectal Carcinoid Study Group.Carcinoid tumors of the rectum: a multi-institutional international collaboration Ann Surg 201025205750–755. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson F C, Levy M J, Dozois E J, Larson D W, Wong Kee Song L M, Boardman L A. Endoscopically identified well-differentiated rectal carcinoid tumors: impact of tumor size on the natural history and outcomes. Gastrointest Endosc. 2014;80(01):144–151. doi: 10.1016/j.gie.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Boudreaux J P, Klimstra D S, Hassan M M et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas. 2010;39(06):753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 19.Woltering E, Bergsland E K, Beyer D T . Chicago: Springer; 2017. Neuroendocrine Tumors of the Jejunum and Ileum. AJCC Cancer Staging Manual, 8th ed; p. 375. [Google Scholar]
- 20.Woltering E, Bergsland E K, Beyer D T . Chicago: Springer; 2017. Neuroendocrine Tumors of the Appendix. AJCC Cancer Staging Manual, 8th ed; p. 389. [Google Scholar]
- 21.Shi C WE, Woltering E, Beyer D T . Chicago: Springer; 2017. Neuroendocrine Tumors of the Colon and Rectum. AJCC Cancer Staging Manual, 8th ed; p. 395. [Google Scholar]
- 22.Mani S, Modlin I M, Ballantyne G, Ahlman H, West B. Carcinoids of the rectum. J Am Coll Surg. 1994;179(02):231–248. [PubMed] [Google Scholar]
- 23.Naunheim K S, Zeitels J, Kaplan E L et al. Rectal carcinoid tumors--treatment and prognosis. Surgery. 1983;94(04):670–676. [PubMed] [Google Scholar]
- 24.Fahy B N, Tang L H, Klimstra D et al. Carcinoid of the rectum risk stratification (CaRRS): a strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14(02):396–404. doi: 10.1245/s10434-006-9197-3. [DOI] [PubMed] [Google Scholar]
- 25.Goebel S U, Serrano J, Yu F, Gibril F, Venzon D J, Jensen R T. Prospective study of the value of serum chromogranin A or serum gastrin levels in the assessment of the presence, extent, or growth of gastrinomas. Cancer. 1999;85(07):1470–1483. [PubMed] [Google Scholar]
- 26.Tormey W P, FitzGerald R J. The clinical and laboratory correlates of an increased urinary 5-hydroxyindoleacetic acid. Postgrad Med J. 1995;71(839):542–545. doi: 10.1136/pgmj.71.839.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi K, Katsumata T, Yoshizawa S et al. Indications of endoscopic polypectomy for rectal carcinoid tumors and clinical usefulness of endoscopic ultrasonography. Dis Colon Rectum. 2005;48(02):285–291. doi: 10.1007/s10350-004-0765-y. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed A, Turner G, King B et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16(03):885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]
- 29.Norlén O, Stålberg P, Öberg K et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36(06):1419–1431. doi: 10.1007/s00268-011-1296-z. [DOI] [PubMed] [Google Scholar]
- 30.Condron M E, Pommier S J, Pommier R F. Continuous infusion of octreotide combined with perioperative octreotide bolus does not prevent intraoperative carcinoid crisis. Surgery. 2016;159(01):358–365. doi: 10.1016/j.surg.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 31.Kinney M A, Warner M E, Nagorney D M et al. Perianaesthetic risks and outcomes of abdominal surgery for metastatic carcinoid tumours. Br J Anaesth. 2001;87(03):447–452. doi: 10.1093/bja/87.3.447. [DOI] [PubMed] [Google Scholar]
- 32.Massimino K, Harrskog O, Pommier S, Pommier R. Octreotide LAR and bolus octreotide are insufficient for preventing intraoperative complications in carcinoid patients. J Surg Oncol. 2013;107(08):842–846. doi: 10.1002/jso.23323. [DOI] [PubMed] [Google Scholar]
- 33.Rinke A, Müller H H, Schade-Brittinger C et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 34.Caplin M E, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(16):1556–1557. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 35.Koizumi M, Onda M, Tanaka N, Seya T, Yamada T, Takahashi Y. Antiangiogenic effect of octreotide inhibits the growth of human rectal neuroendocrine carcinoma. Digestion. 2002;65(04):200–206. doi: 10.1159/000063822. [DOI] [PubMed] [Google Scholar]
- 36.Höpfner M, Schuppan D, Scherübl H. Treatment of gastrointestinal neuroendocrine tumors with inhibitors of growth factor receptors and their signaling pathways: recent advances and future perspectives. World J Gastroenterol. 2008;14(16):2461–2473. doi: 10.3748/wjg.14.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J C, Fazio N, Singh Set al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study Lancet 2016387(10022):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strosberg J, El-Haddad G, Wolin E et al. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(02):125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]


