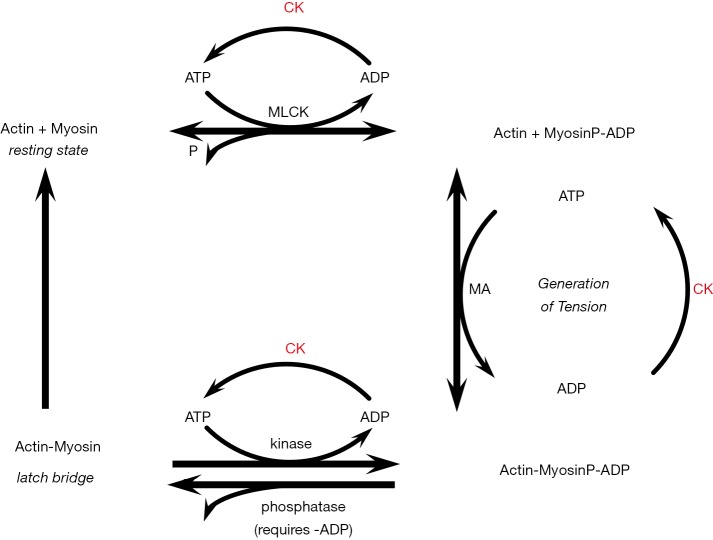
Figure 1.
Smooth muscle contraction with high creatine kinase activity. A model of the four-state contractile system of vascular smooth muscle adapted from Brewster (18), based on Murphy (25), and Hai and Murphy (26). Ca2+ initiates contraction with activation of MLCK. MLCK phosphorylates myosin light chains to activate myosin ATPase (MA) and the MyosinP-ADP interacts generates tension with actin through an Actin-MyosinP-ADP complex. Shortening occurs through cross bridge cycling. The MyosinP-ADP can be dephosphorylated to form the so-called “latch bridges”, which are slowly cycling cross bridges depending on ADP (27). Controlled exit from the latch bridge occurs by re-phosphorylation of myosin, while dephosphorylation of MyosinP and a decrease in Ca2+concentration inactivates the high-tension state. Smooth muscle creatine kinase (CK) activity is relatively low compared to striated muscle (23). With relatively high CK activity, lower ADP levels might hinder the ADP-dependent latch bridge formation and enhance myosin ATPase activity, leading to greater microvascular contractility (18). MLCK, myosin light chain kinase.