Figure 4.
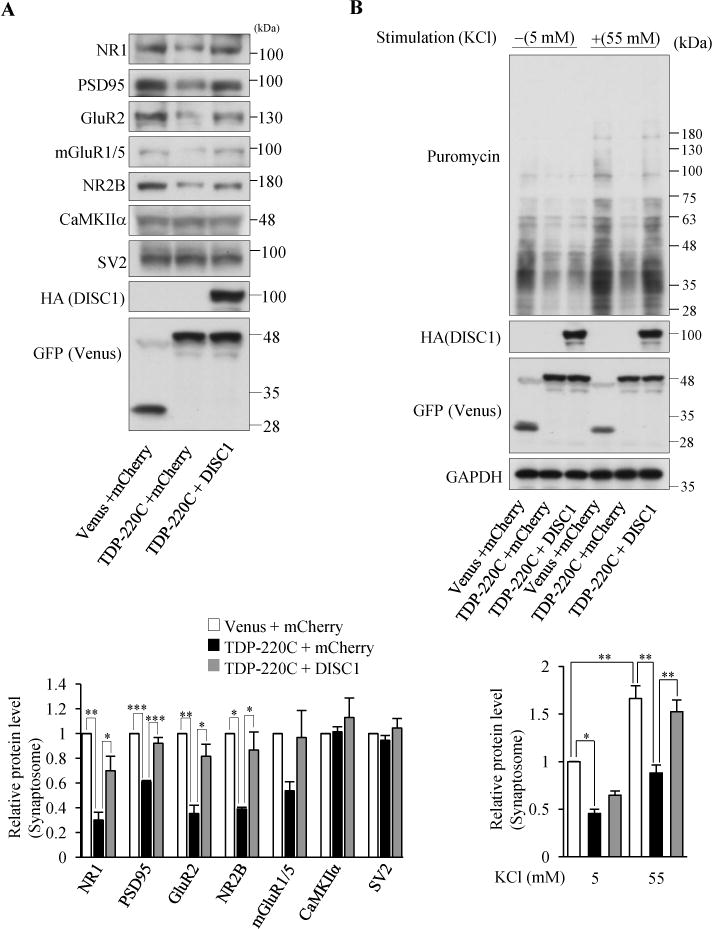
Co-aggregation of TDP43 and DISC1 impairs DISC1-mediated local translation. (A) Indicated synaptic proteins in the synaptosomal fraction of cultured cortical neurons infected with indicated lentivirus were detected by western blotting (top). The levels of synaptic proteins relative to those in control neurons are shown (bottom), (n=3 for NR2B n=4 for rest of genes, NR1: F(2,9)=15.60, P=0.0012; PSD95: F(2,9)=41.39, P<0.0001; GluR2: F(2,9)=16.17, P=0.0010; NR2B: F(2,9)=10.50, P=0.0110, one-way ANOVA; *P<0.05, **P<0.01, ***P<0.001, Bonferroni’s multiple comparison test post hoc). (B) Co-expression of DISC1 restored the decrease in neural stimulation-dependent protein synthesis by TDP-220C aggregation. Cultured cortical neurons were stimulated with 55 mM KCl, followed by the treatment with 10 μg/ml puromycin. The puromycin-labeled proteins in the isolated synaptosomal fraction were detected by western blotting with an anti-puromycin antibody. The signal intensities of puromycin-incorporated polypeptides were normalized to those of GAPDH and then expressed as the relative ratio of control neurons (right) (n=4, F(5,18)=18.99, P<0.0001, one-way ANOVA; *P<0.05, **P<0.01, Bonferroni’s multiple comparison test post hoc). Throughout the figures, error bars represent S.E.M.
