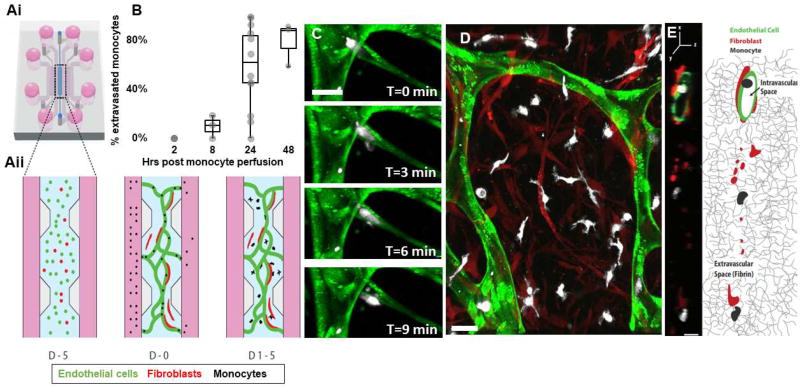
Figure 1. Monocytes transmigrate through the microvascular network over time.
A)i-Schematic of the microfluidic device, showing the central compartment shaded in blue that contains the cells surrounded by the microfluidic channels filled with media (pink), ii- Close-up of the central region containing the cells mixed in a 3D hydrogel at different time points: at day −5, endothelial cells (green) and fibroblasts (red) are suspended in a 3D fibrin gel. 5 days later, at day 0, endothelial cells have connected and formed a network with lumens open to the flanking microfluidic channels. Monocytes are isolated from blood and perfused through the networks thanks to a transient pressure drop (ΔP) established across the central region. Within a few days (D2-4) monocytes have transmigrated through the endothelial wall and are found in the extracellular fibrin gel around the networks, interacting with fibroblasts. B) Quantification of monocyte extravasation 0, 8, 24 and 48 hrs after monocyte perfusion in the microvascular network. Each data point represents the percentage of extravasated monocyte in one device. N=3–15 devices per condition, 1–9 donors per condition. C) Confocal images of a monocyte (white) undergoing extravasation through the endothelium (green). Bar is 20 µm. D) Representative image of the monocytes 4 days after perfusion. Most have extravasated and are in the extravascular space, in close interaction with fibroblasts (red). Bar is 20 µm. E) Representative cross-sectional view of a vessel segment (green) and the extravascular space surrounding it, as observed with confocal microscopy and schematically represented (right panel). Fibroblasts (red) are found in the extravascular space within the 3D fibrin matrix, while monocytes (white) can be found either inside the hollow vessels or outside in the 3D fibrin matrix. Bar is 10 µm.