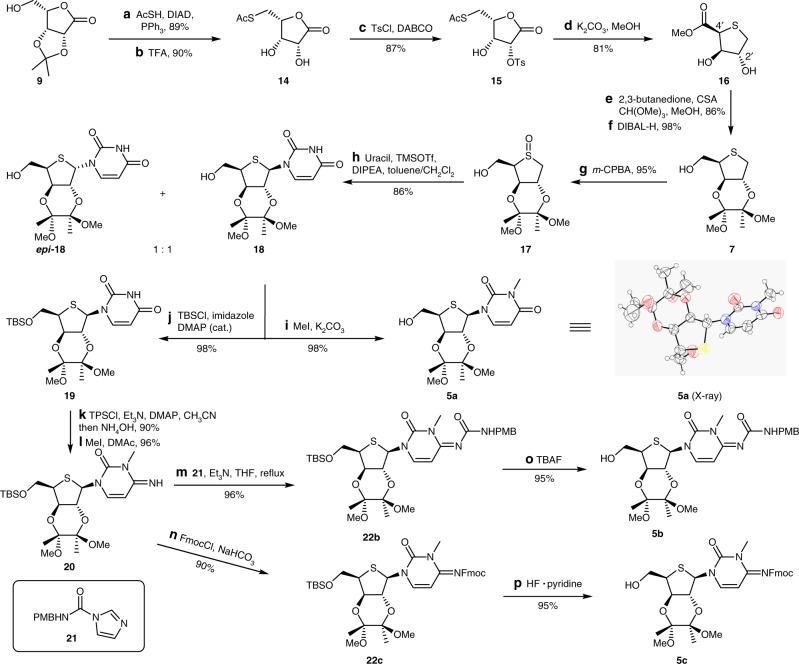
Fig. 3.
Synthesis of intermediates 5a-c and X-ray crystal-structure diagram of 5a. Reagents and conditions: a AcSH (2.2 equiv), DIAD (2.2 equiv), PPh3 (2.2 equiv), THF, 0 °C, 5 h, 89%; b TFA, CH2Cl2, H2O, 5 h, 90%; c TsCl (1.2 equiv), DABCO (1.1 equiv), CH3CN, 1 h, 87%; d K2CO3 (1.5 equiv), MeOH, −30 °C, 3 h, 81%; e 2,3-butanedione (1.2 equiv), trimethoxymethane (4.0 equiv), CSA (0.1 equiv), MeOH, reflux, 24 h, 86%; f DIBAL-H (2.1 equiv), CH2Cl2, 0 °C, 2 h, 98%; g m-CPBA (1.0 equiv), CH2Cl2, 0 °C, 0.5 h, 95%; h uracil (2.0 equiv), TMSOTf (8.0 equiv), DIPEA (8.0 equiv), toluene, CH2Cl2, 3 h, d.r. 1:1, 86%; i methyl iodide (1.2 equiv), K2CO3 (1.5 equiv), DMF, 2 h, 98%; j TBSCl (2.2 equiv), imidazole (2.2 equiv), DMAP (0.1 equiv), CH3CN, 2 h, 98%; k TPSCl (2.0 equiv), Et3N (2.0 equiv), DMAP (2.0 equiv), CH3CN, 1 h, then NH4OH, 20 h, 90%; l methyl iodide (3.0 equiv), DMAc, 3.5 h, 96%; m 21 (2.0 equiv), Et3N (1.0 equiv), THF, reflux, 96%. n Fmoc-Cl (2.0 equiv), NaHCO3 (4.0 equiv), THF/H2O, 90%; o TBAF (1.3 equiv), THF, 2 h, 95%; p HF·pyridine (5.0 equiv), THF, 36 h, 95%. AcSH thiolacetic acid, DIAD diisopropyl azodiformate, DABCO 1,4-diazabicyclo[2.2.2]octane, CSA camphorsulfonic acid, DIBAL-H diisobutylaluminum hydride, TPSCl 2,4,6-triisopropylbenzenesulfonyl chloride, DMAP 4-dimethylaminopyridine, DMAc N, N-dimethylacetamide, TBAF tetrabutylammonium fluoride