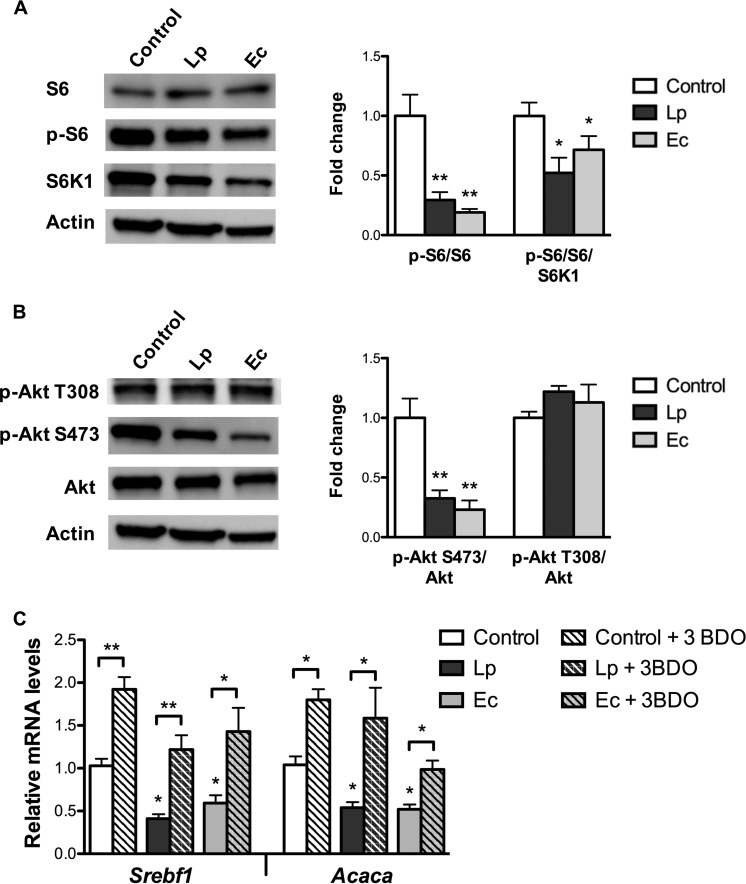
FIG 4 .
L. paracasei (Lp) and E. coli (Ec) inhibit the Akt/mTOR pathway in cultured enterocytes. m-ICcl2 cells were polarized on transwell inserts for 14 to 21 days before infection of the upper compartment with bacteria (MOI of 100); cell lysates were recovered following 16 h of infection. Control: non-exposed cells. (A and B) Western blot analysis of (A) S6 signaling and (B) Akt signaling. Cell lysates were subjected to Western blot analysis for (A) phosphorylated and total endogenous S6 and S6K1 and for (B) phosphorylated and total endogenous Akt. Representative sample blots and quantifications of S6 and Akt phosphorylation levels are shown. Values are presented as ratios between levels of phosphorylated protein S6 (A) or Akt and total endogenous protein (B), normalized to control cells. (C) mRNA levels of Srebf1 and Acaca assessed by RT-qPCR using the Actin gene as the reporter gene following 16 h of coincubation with bacteria and with the mTOR activator 3-BDO. Results are expressed as means of fold change ± SEM. Statistical significance is expressed relative to the control unless otherwise specified. *, P < 0.05; **, P < 0.01 (one-way ANOVA). A total of 3 experiments were performed in duplicate.