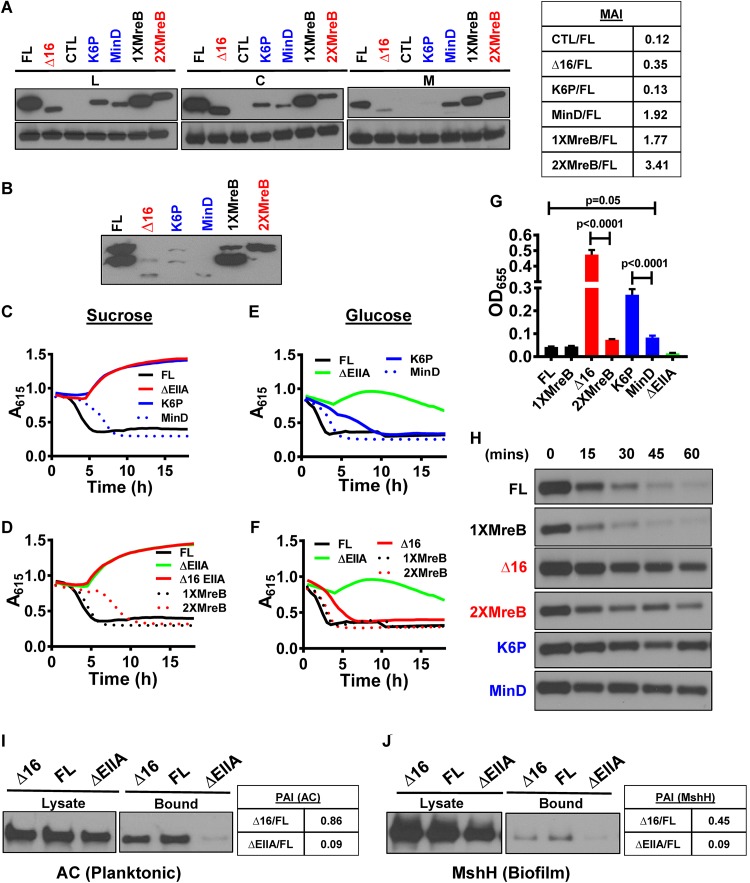
FIG 4 .
AH swapping establishes that the function of the V. cholerae EIIAGlc AH is not amino acid sequence dependent. (A) Anti-V5 antibody immunoblot assays of the lysate (L), cytoplasmic (C), and membrane (M) fractions of V. cholerae expressing the indicated affinity-tagged EIIAGlc constructs. The membrane association index (MAI) with respect to FL is given at the right. (B) Phos-tag acrylamide gel electrophoresis and anti-V5 antibody immunoblot assay of V. cholerae expressing the indicated EIIAGlc alleles. A615 measured over time in cultures of V. cholerae expressing FL-EIIAGlc (FL), K6P-EIIAGlc (K6P), 1X-MreB EIIAGlc (1XMreB), 2X-MreB EIIAGlc (2XMreB), EIIAGlc-MinD (MinD), and ΔEIIAGlc (ΔEIIA). (C to F) Minimal medium supplemented with pH indicators and sucrose (C and D) or glucose (E and F) was used. Results are representative of three independent experiments. (G) Quantification of biofilm formation by strains as indicated in minimal medium supplemented with glucose. Bars represent the mean from biological triplicates. Error bars indicate the standard deviation. Statistical significance was calculated using a one-way analysis of variance followed by Tukey’s multiple-comparison test. (H) Northern blot of the sRNA csrB in LB-cultured, mid-log-phase V. cholerae cells that express the indicated EIIAGlc alleles. Samples were harvested at the noted times after addition of rifampin to arrest transcription elongation. (I and J) Immunoblots of adenylate cyclase (AC) (I) and MshH (J) in cell lysates and the bound fractions that coimmunoprecipitated with FL EIIAGlc or Δ16 EIIAGlc. The partner association index (PAI) with respect to FL is given at the right.