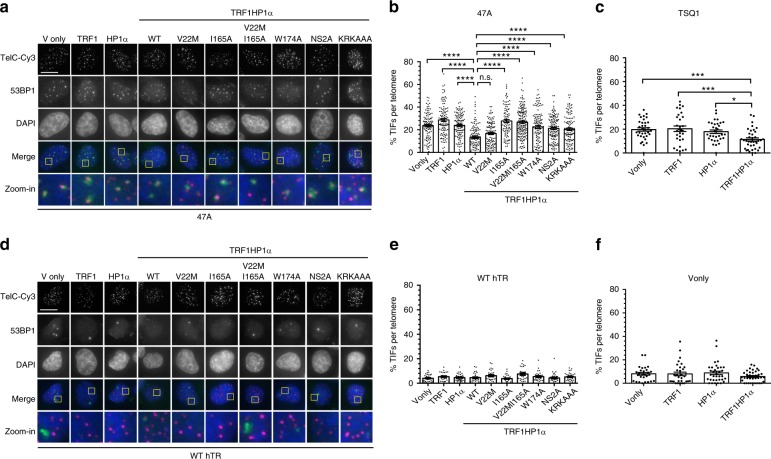
Fig. 4.
TRF1HP1α results in reduced TIFs induced by mutant hTR expression. Cells stably expressing TRF1HP1α (WT or mutant variants of HP1α) were infected with lentivirus containing WT hTR, or mutant hTR (47A or TSQ1) on day 0, selected for stable expression after 48 h, and analyzed on day 5. a Fluorescence microscopy images of representative cells expressing mutant hTR 47A stained with telomeric (Tel-Cy3) peptide nucleic acid (PNA) probes (magenta in merged image) via fluorescent in situ hybridization (FISH), antibody against DNA-damage repair protein marker 53BP1 (green in merged image), and counterstained with DAPI. Zoom-in images (the last row) correspond to yellow-squared regions of the row above. b % TIF per telomere of each nucleus is quantified; n = 94–159 nuclei combining data of three independent experiments. ****p < 0.0001; n.s. (no significance). a, b Scale bar: 10 µm. c Upon TSQ1 expression, TRF1HP1α results in fewer TIFs compared to Vonly, TRF1 or HP1α controls. TRFHP1α ~11.4% shows decreased TIFs compared to Vonly: ~19.7% ***p = 0.0008; TRF1: ~20.4% ***p = 0.0005; HP1α: ~17.9% *p = 0.0137 (n = 30–38 nuclei). b, c Significance is assessed by one-way ANOVA and Dunnett’s multiple comparison test with 95% confidence level. d Fluorescence images of control cells overexpressing WT hTR (n = 27–36 nuclei). Same color scheme as a. TIFs quantification in the presence of e WT hTR or f Vonly (n = 28–36 nuclei) show minimal baseline DNA damage at telomeres. b, c; e, f Error bars represent s.e.m.