Fig. 3.
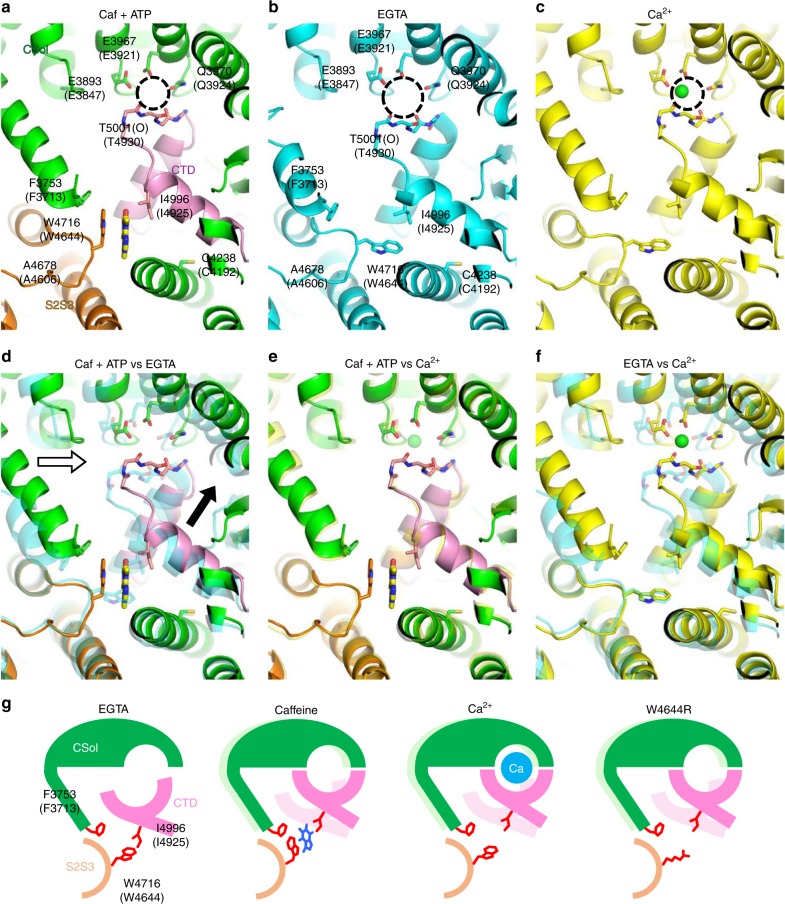
Conformational changes of Ca2+-binding and caffeine-binding sites in the presence and absence of ligands. a–c The architecture of putative Ca2+-binding and caffeine-binding sites under different conditions. Structures in the presence of (a) caffeine and ATP (Caf + ATP) (PDB accession code: 5TAP), (b) EGTA (5TB0) and (c) Ca2+ (5T15) are depicted in ribbon representation. The Ca2+-binding pocket is indicated by a dotted circle. In Caf + ATP, the core solenoid (CSol) domain, S2S3 domain, and CTD are colored in green, orange, and pink, respectively. The likely interacting residues of the putative Ca2+-binding and caffeine-binding sites of rabbit RyR1 and mouse RyR2 (in parentheses) are labeled. d–f Structures in two different conditions are overlaid: (d) Caf + ATP and EGTA, (e) Caf + ATP and Ca2+, and (f) EGTA and Ca2+. d In Caf + ATP, CSol moves a ~2 Å rightward (white arrow) and CTD moves a ~2 Å upward (black arrow) compared with in EGTA. e Conformational changes by Caf + ATP closely resemble those by Ca2+. f Similar movements of CSol and CTD were observed by Ca2+. g Hypothetical conformational changes of Ca2+-binding and caffeine-binding sites in RyR2 by interaction between tryptophan and isoleucine. In EGTA, W4644, and I4925 interact to pull the CTD toward the S2S3 domain, which makes the Ca2+-binding pocket larger and less favorable for Ca2+. Caffeine breaks the interaction by rotating the tryptophan side chain (Caf + ATP). This moves the CTD toward the CSol to make the Ca2+-binding pocket smaller and more favorable for Ca2+. Similar conformational changes occur in response to Ca2+. Mutation in tryptophan (W4644R) may also break the interaction to cause an upward shift of the CTD, resulting in enhanced Ca2+ sensitivity. Light colors in caffeine, Ca2+, W4644R indicate locations of the CSol and CTD in the EGTA state