Fig. 7.
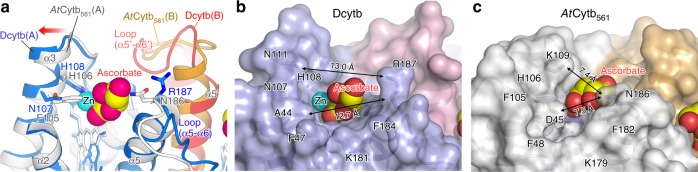
Comparison of the binding pockets on the apical surfaces of Dcytb and AtCytb561. a Superposition of Dcytb and AtCytb561. The α3 helix is bent in the middle to accommodate binding of Zn2+ and ascorbate. The loop connecting α5–α6 contributes to this binding pocket and exhibits significant conformational difference between the two proteins. Dcytb (A-chain (blue), B-chain (red)) and AtCytb561 (A-chain (gray), B-chain (orange)) are colored as indicated. The A-chains of the two proteins were superimposed for calculations. Space filling models are shown for Zn2+ and ascorbate bound to Dcytb. The α2 helices of the A-chains of both proteins are excluded from this view for clarity. b The binding pocket of Dcytb is wide and open above the ligand. This view is the same as shown in a. c Ascorbate in the binding pocket of AtCytb561 is more buried