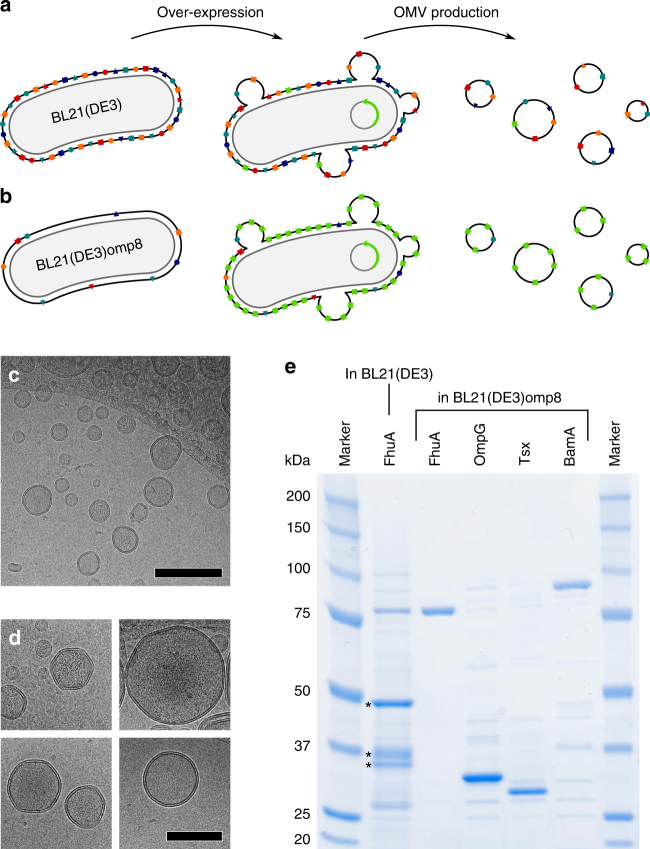
Fig. 1.
Preparation and analysis of outer membrane protein (Omp)-enriched outer membrane vesicles (OMVs). a, b To characterize the folding and structure of outer membrane proteins (OMPs) in their native environment we prepared outer membrane vesicles (OMVs) from two different E. coli strains. a The widely used E. coli expression strain BL21(DE3) contains large quantities of four major porins, namely OmpA, OmpF, OmpC, and LamB, which leaves limited space for the overexpression of additional OMPs. b Using strain BL21(DE3)omp8, which lacks the four major porins, allows overexpressing high amounts of selected OMPs. c, d Collected OMVs ranged from 50 to 250 nm in diameter, as observed by cryo-transmission electron microscopy. Shown are OMVs enriched in FhuA, for all other OMVs see Supplementary Fig. 2. d The majority of OMVs appeared unilamellar and intact. Scale bars, 250 nm (b) and 100 nm (d). e SDS-PAGE of OMVs collected after overexpression of FhuA in E. coli BL21(DE3), as well as OMVs collected after overexpression of FhuA, OmpG, Tsx, and BamA, respectively, in E. coli BL21(DE3)omp8. Whereas OMVs from BL21(DE3) cells show large quantities of native porins (marked by asterisks), OMVs from BL21(DE3)omp8 cells show a clear band of the overexpressed protein