Fig. 2.
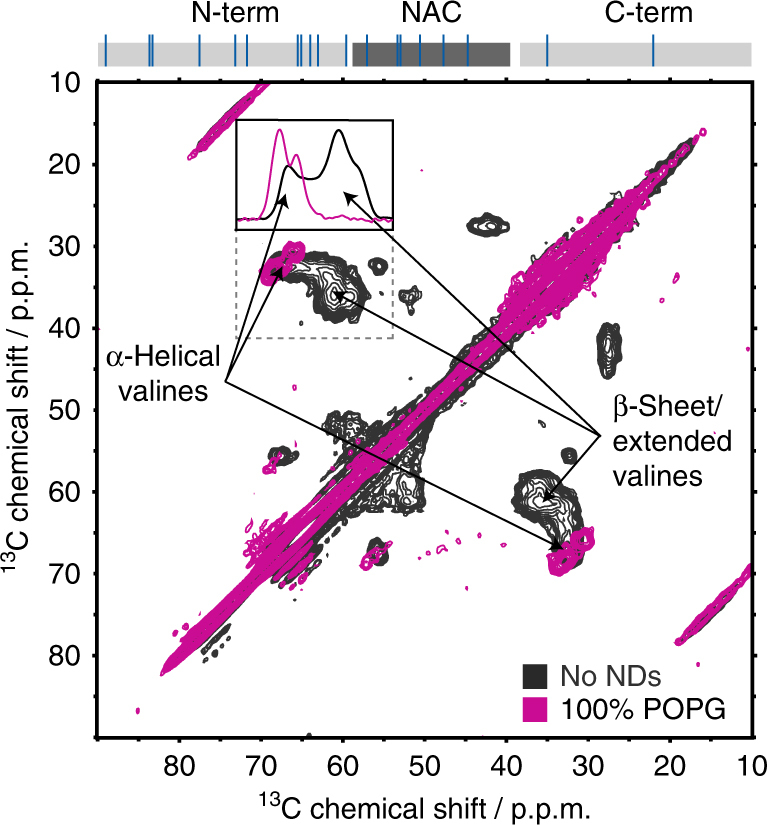
Nanodiscs binding induces α-helical structure in αS. [13C-13C]-Proton driven spin diffusion magic angle spinning-DNP spectra of free non-acetylated αS in frozen solution (black) and when bound to NDs with 100% POPG lipids (purple). Selective isotope labeling was used to specifically monitor valine Cα-Cβ chemical shift distributions. Peak positions indicative of β-sheet and α-helical secondary structure are labelled. The insert shows normalized 1D projections of the highlighted region (dashed square) in the absence (black) and presence of 100% POPG NDs. Signal deconvolution of these spectra reveals that about 92% of the valines are in an α-helical configuration in the presence of 100% POPG NDs. The occurrence of valine residues in the αS sequence is shown on top (blue lines). According to the respective solution NMR attenuation profile (Fig. 1c, purple) 18 out of 19 valines (i.e., 94.7%) are expected in the membrane-bound state at the used conditions
