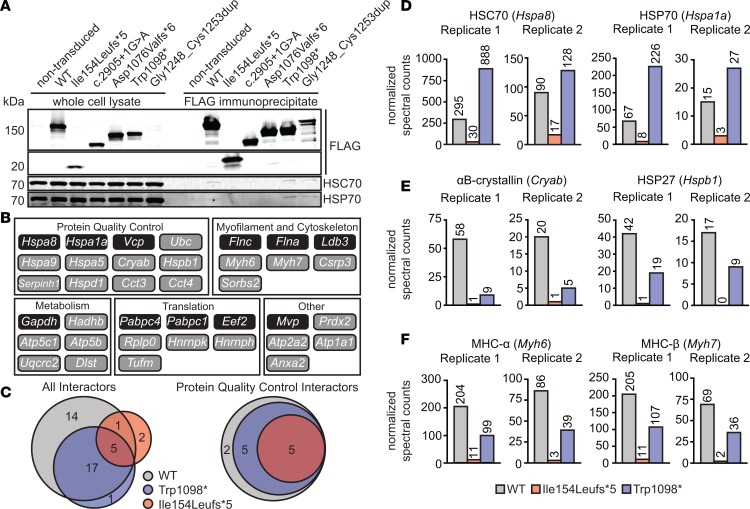
Figure 2. Interactions between MYBPC3 and molecular chaperones.
(A) Western blot showing co-IP of HSC70 and HSP70 with FLAG-tagged WT and mutant MYBPC3. Shorter MYBPC3 truncated proteins show loss of this interaction. (B) Summary of WT MYBPC3–interacting proteins manually categorized by function. All interactors were detected in both co-IP–MS experiments and showed a calculated fold change (FC-A) in spectral counts 2 or higher over nontransduced myocytes in at least one independent experiment. Interactors within dark boxes showed an FC-A of 2 or higher over background in both experiments. (C) Venn diagrams showing overlap of interacting proteins (left, all interactors; right, protein quality control interactors) identified in immunoprecipitates for WT, Ile154Leufs*5, and Trp1098* MYBPC3. MYBPC3 mutants show loss of interaction with protein quality control–related proteins. (D–F) Normalized spectral counts for a subset of MYBPC3-interacting proteins from 2 independent co-IP–MS experiments. Adjusted spectral counts were normalized to abundance of FLAG-MYBPC3 bait protein. (D) HSC70 and HSP70 were more abundant in FLAG-Trp1098* samples, but less abundant in FLAG-Ile154Leufs*5 samples compared with FLAG-WT, while small heat shock proteins (E) showed loss of interaction with both mutants. (F) MYBPC3 mutants also showed reduced association with known binding partners myosin heavy chains α and β.