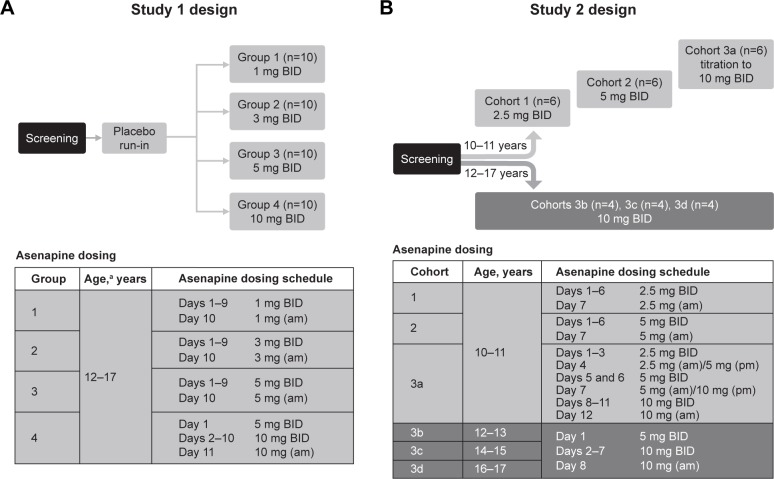
Figure 1.
Design and dosing schedule for (A) study 1 and (B) study 2.
Notes: In study 1, screening was performed within 3 weeks of baseline. Dosing was BID, except for the last day of dosing (only morning dose administered). In study 2, screening was performed within 28 days prior to baseline. Sublingual asenapine was given BID. Cohort 3a was originally scheduled similarly to cohorts 3b–3d (5 mg BID on day 1 followed by 10 mg BID thereafter); however, after safety results of cohort two became available, a protocol amendment modified the original treatment regimen (slower titration to 10 mg asenapine) to improve tolerability. a≥4 subjects per group must have been between 12 and 15 years of age.
Abbreviation: BID, bis in die (twice daily).