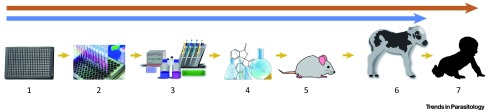
Figure 3. A drug-discovery screening pipeline for Cryptosporidium.
(1) A high content imaging infection assay in vitro was used to identify Cryptosporidium parvum inhibitory compounds. (2) Secondary screening using a cytopathic effect-based assay was used to identify imidazopyrazines and pyrazolopyridines with inhibitory activity against C. parvum and Cryptosporidium hominis. (3) Identification, expression, and enzymatic activity of the C. parvum phosphatidylinositol-4-OH kinase (PI4K). (4) Pharmacokinetics and toxicity testing of KDU731. (5) Activity of KDU731 against transgenic C. parvum infection in interferon-gamma knockout mice. (6) Activity of KDU731 against native C. parvum infection in the neonatal calf model. (7) Additional preclinical evaluation is needed before initiation of human clinical trials. Figure reproduced with permission from Elsevier 42.