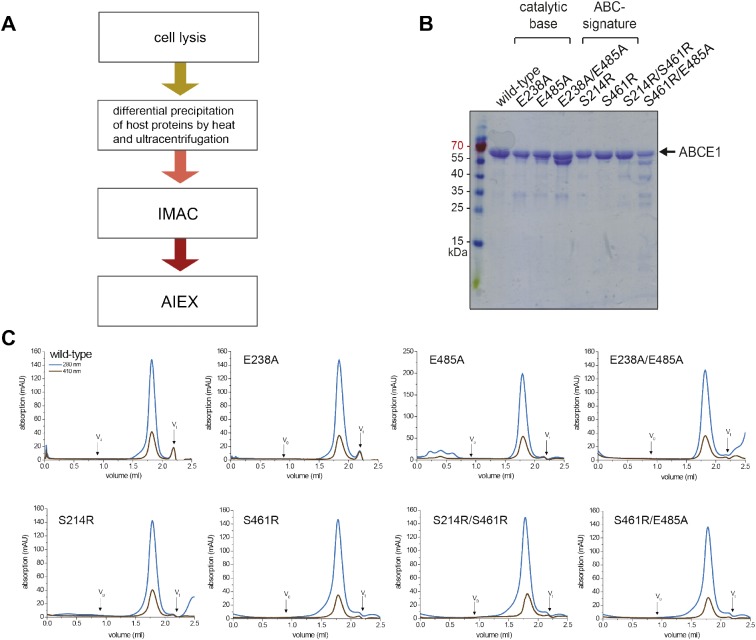
Figure S1. Protein purification and quality control.
(A) All splitting factors were purified from E. coli in a three-step process after lysis; differential precipitation at 65°C removed most host proteins. In a second purification step, ABCE1 was isolated via a C-terminal His6 affinity tag by IMAC. ABCE1 with disassembled FeS clusters and most degradation products were removed by subsequent AIEX. (B) Purified ABCE1 was analyzed by SDS–PAGE (12.5%, Coomassie staining). Some of the mutants show degradation products. (C) In SEC, all ABCE1 variants eluted in a single peak as seen by absorption at 280 nm (blue). Additional absorption at 410 nm (brown) demonstrates an assembled iron-sulfur cluster. SEC was performed in 20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, and 1 mM DTT on a Superose 6 2.4 ml analytical grade column (GE Healthcare) applying 20–30 μg of protein.