Figure 1.
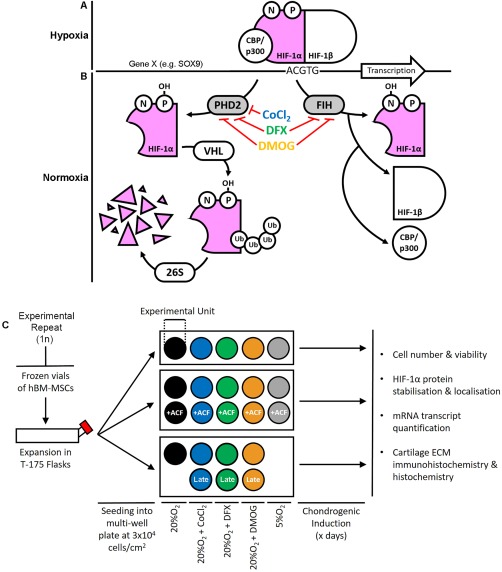
Schematics highlighting the role of hydroxylase inhibitors in regulation of HIF‐1α‐mediated transcription and the study experimental design. (A): Under hypoxic conditions, HIF‐1α forms an active transcription complex with HIF‐1β and co‐factors such as CBP/p300. This HIF complex then binds to the promoter regions of target genes at the HIF‐response element sites, inducing transcription. (B): At normoxia, two hydroxylases—PHD2 and FIH, utilize oxygen and other substrates to hydroxylate HIF‐1α which promotes its degradation and inhibits binding by CBP/p300. Here, we aimed to stabilize HIF‐1α at normoxia by inhibiting the hydroxylases with CoCl2, DFX or DMOG. (C): Experiment design. To produce each biological replicate, hBM‐MSCs were thawed and expanded to passage 5 before re‐seeding at a density of 3 × 104 cell per cm2 in multi‐well plates. Each well or set of wells was assigned to a specific condition: 20%O2, 20%O2+CoCl2, 20%O2+DFX, 20%O2+DMOG, or 5%O2. Separate experiments included each HIF‐stabilizing compound in the presence or absence of ACF, and a comparison of late with constitutive exposure. In each condition, cultures were chondrogenically differentiated before assays at the time points specified in the legend of each figure. Abbreviations: ACF, Acriflavine; CoCl2, cobalt chloride; DFX, desferrioxamine; DMOG, dimethyloxalylglycine; ECM, extracellular matrix; FIH, factor inhibiting hypoxia inducible factor; hBM‐MSCs, human bone marrow‐derived mesenchymal stem cells; HIF, hypoxia inducible factor; PHD2, prolyl hydroxylase 2.
