Chinese fossils reveal a Triassic insect radiation.
Abstract
The Triassic represented an important period that witnessed the diversification of marine and terrestrial ecosystems. The radiations of terrestrial plants and vertebrates during this period have been widely investigated; however, the Triassic history of insects, the most diverse group of organisms on Earth, remains enigmatic because of the rarity of Early-Middle Triassic fossils. We report new insect fossils from a Ladinian deposit (Tongchuan entomofauna) dated to approximately 238 to 237 million years ago and a Carnian deposit (Karamay entomofauna) in northwestern China, including the earliest definite caddisfly cases (Trichoptera), water boatmen (Hemiptera), diverse polyphagan beetles (Coleoptera), and scorpionflies (Mecoptera). The Tongchuan entomofauna is near the Ladinian-Carnian boundary in age, providing a calibration date for correlation to contemporaneous biotas. Our findings confirm that the clade Holometabola, comprising most of the modern-day insect species, experienced extraordinary diversification in the Middle-Late Triassic. Moreover, our results suggest that the diversification of aquatic insects (a key event of the “Mesozoic Lacustrine Revolution”) had already begun by the Middle Triassic, providing new insights into the early evolution of freshwater ecosystems.
INTRODUCTION
The end-Permian mass extinction (EPME) caused a severe crisis in terrestrial ecosystems, possibly due to global wildfires at the Permian-Triassic boundary, long-term aridification, and short-term warming and acid rain during the Early Triassic (1, 2). After the EPME, the Triassic is a period for the radiation of organisms in both marine and terrestrial ecosystems (2, 3); it marks the first major step in the origin of modern ecosystems and is thus frequently known as the “Dawn of the Modern World” (4, 5). For terrestrial ecosystems, many key modern vertebrates appeared during the Triassic (2), including two of the most important events: the origin and rise of the dinosaurs (6) and mammaliaforms (7). Plants experienced an especially important development during this period. Following the domination of the lycopsid plants during the Early Triassic, conifers, cycadophytes, and pteridosperms all radiated during the Middle Triassic, subsequently evolving into their modern forms (8, 9).
The EPME led to changes in insect fauna at high taxonomic levels: a drop in abundance and overall diversity (10–12) and probably a severe extinction (13, 14). After the EPME, Triassic insects probably kept pace with the megafloral development: Modern insects feeding on the pteridophytes and gymnosperms replaced ancient insects feeding on the pteridophytes and basal gymnosperms (15); plant-insect associations became significantly diverse during the Late Triassic (16–18). However, the scarcity of Early-Middle Triassic entomofaunas constrains our knowledge on the radiation of insects (19–21). In contrast to terrestrial plants and vertebrates, the evolutionary history of Triassic insects is still poorly understood. Little is known about the Triassic evolution of holometabolous and aquatic insects. These groups have the potential to provide insights into the early evolution of terrestrial (including freshwater) ecosystems (22, 23) because the former is the most species-rich group of extant animals (24) and the latter is a key element of the “Mesozoic Lacustrine Revolution” (25).
Here, we report a late Ladinian Tongchuan entomofauna, with radioisotopically determined ages, and a Carnian Karamay entomofauna from northwestern China. Our results provide not only the earliest records of several modern insect elements but also a calibration point for correlation with contemporaneous biotas. These findings confirm that holometabolous and aquatic insects experienced extraordinary diversification during the Middle-Late Triassic.
RESULTS
Insect fossils
More than 800 insect fossils were collected from the upper part of the Karamay Formation in the Huayuangou outcrop (45°40′24″N, 84°55′57″E), Karamay City, Xinjiang (Fig. 1, A, C, and E), and the top of the lower part of the Tongchuan Formation in the Qishuihe outcrop (28°17′24″N, 117°52′32″E), Hejiafang Village, Jinsuoguan Town, Tongchuan City, Shaanxi Province (Fig. 1, B, D, and E, and fig. S1), Northwest China. The insect-bearing layer of the Tongchuan Formation is considered to be from the latest Ladinian stage [~238 to 237 million years (Ma) ago] and that of the Karamay Formation is regarded as Carnian in age. For detailed stratigraphic information, see the Supplementary Materials.
Fig. 1. Geology of Tongchuan and Karamay entomofaunas.
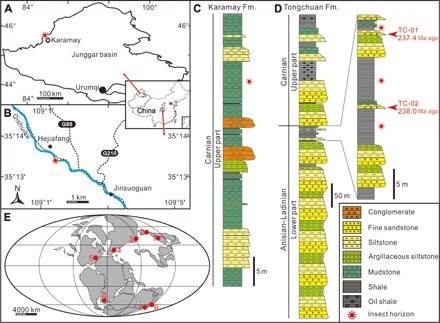
Geological map showing the location of entomofauna in Karamay City in (A) Xinjiang and (B) Tongchuan City in Shaanxi, Northwest China. (C and D) Stratigraphic column showing lithologies, sample points, and age results. Global paleogeographic reconstructions [Carnian; after Rasnitsyn and Quicke (33) and Golonka (62)], highlighting main coeval insect–bearing localities (E): (1) Tongchuan, China (Ladinian); (2) Karamay, China (Carnian); (3) Madygen, Kyrgyzstan (Ladinian-Carnian); (4) Vosges, France (early Anisian); (5) Solite, USA (late Carnian); (6) Ipswich, Australia (Carnian); (7) Brookvale, Australia (Anisian); and (8) Molteno, South Africa (Carnian).
U-Pb geochronology
Two tuffaceous sandstone samples (TC-01 and TC-02), each weighing about 5 kg, were collected between insect fossil–bearing strata of the Qishuihe outcrop (Fig. 1D) and separated for LA-MC-ICP-MS (laser ablation–multi-collector–inductively coupled plasma mass spectrometry) U-Pb dating. The zircon grain sizes of sample TC-01 (Fig. 2, A and B) ranged from 80 to 120 μm. Most grains exhibited euhedral morphologies and oscillatory zoning patterns, indicating an igneous origin (Fig. 2B). Angular grain facets also suggest minimal abrasion during sedimentary transport and support the interpretation that the tuffaceous sandstone depocenter lays near the magmatic source of the zircons. Eighty-nine analyses provided concordant ages ranging from 2480 to 237 Ma ago (Fig. 2B). Eight youngest analyses gave a weighted mean of 237.4 ± 0.9 Ma ago [mean square weighted deviation (MSWD), 0.4; uncertainties are given at the 2σ level] as the maximum depositional age for sample TC-01. The major subpopulation of ages generally fell between 237 and 362 Ma ago, with some between 1538 and 2480 Ma ago. Several sporadic ages fell in the Devonian (404 Ma ago), Ordovician (472 Ma ago), and Cambrian (492 Ma ago). The zircon grain sizes of sample TC-02 (Fig. 2, C and D) were similar to those from sample TC-01. While some grains were intact, most exhibited fractured morphologies. Euhedral facets and oscillatory zoning patterns indicate an igneous origin and limited exposure to sedimentary processes (Fig. 2D). Ninety-four zircon grains gave concordant ages ranging from 2703 to 237 Ma ago (Fig. 2D). The seven youngest, with equivalent 206Pb/238U dates, gave a weighted mean of 238.0 ± 1 Ma ago (MSWD, 0.8; uncertainties are given at the 2σ level) as the maximum depositional age for sample TC-02. The largest subpopulation of ages generally fell between 237 and 307 Ma ago, and the second one fell between 1611 and 2703 Ma ago. The age distribution also included one outlier age of 503 Ma ago (Cambrian). The two youngest age populations with weighted means of 237.4 ± 0.9 Ma ago and 238.0 ± 1 Ma ago suggest a latest Ladinian age (26) for the top of the lower part of the Tongchuan Formation. For detrital zircons, the youngest age populations may not represent the most accurate depositional age (27), since the deposition should be later than youngest magmatic events (28, 29), but may offer approximate constraints on deposition (6). The latest Ladinian age agrees with the previous isotopic age results (~236 to 234 Ma ago) (30) for the upper part of the Tongchuan Formation and the biostratigraphic correlation (Middle Triassic) for this formation and can approximately represent the depositional age (for detailed age discussions, see the Supplementary Materials).
Fig. 2. U-Pb geochronology for samples from layers bearing Tongchuan entomofauna.
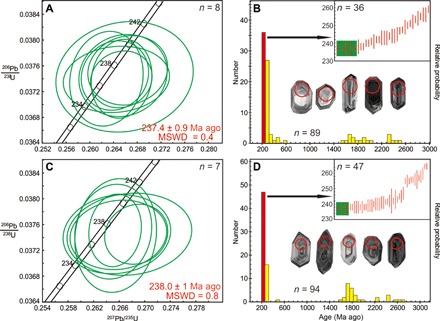
U-Pb concordia plots for zircons in youngest ages (left) and frequency histograms (right) of detrital age populations from sample TC-01 (A and B) and sample TC-02 (C and D). Black arrows showing rank order plots (green bars represent youngest ages). Select cathodoluminescence (CL) images of zircons are in frequency histograms (age, 237 Ma ago; red circle diameter, 40 μm).
DISCUSSION
Characters of two entomofaunas
The Tongchuan entomofauna contains at least 28 insect families in 11 orders, viz. Blattodea, Coleoptera, Diptera, Grylloblattida, Glosselytrodea, Hemiptera, Mecoptera, Miomoptera, Odonatoptera, Orthoptera, and Trichoptera (table S1), making it among the most diverse Triassic entomofaunas (typical coeval insect–bearing sites; see Fig. 1E). This entomofauna includes more than 14 families of holometabolous insects, which also distinctly outnumber other insects with respect to specimen numbers (approximately 65% of 520 specimens). The Tongchuan entomofauna has many typical Triassic elements, such as Zygophlebiidae (Odonatoptera), Locustavidae (Orthoptera), and Curvicubitidae (Hemiptera), which were widespread in the Ladinian-Carnian of Kyrgyzstan and the Carnian of Ipswich, Australia (31). It yields some Late Permian and Early Triassic components, such as Chaulioditidae (Grylloblattida: Chauliodites) (21), Surijokocixiidae (Hemiptera), Dunstaniidae (Hemiptera), and primitive Aphidoidea (Hemiptera), and the Aphidoidea is related to a Permian aphid from France (32). In addition, it yields the earliest Cicadocorinae (Hemiptera) (Fig. 3I), which are common moss bugs in the Jurassic and Early Cretaceous (33). The Karamay entomofauna yields 10 insect families in six orders, including caddisfly cases and the earliest known water boatmen. Aquatic beetles and bugs (Permosynidae, Schizocoleidae, and Corixidae) are quite abundant in this entomofauna, in which the diversity is dominated by beetles (five families). The complete and detailed descriptions of these fossils from the two entomofaunas will be published elsewhere.
Fig. 3. Representative fossils from Tongchuan and Karamay entomofaunas.

Tongchuan entomofauna (A to C, E to J, and L to O), and Karamay entomofauna (D and K). (A) Zygophlebia (Odonatoptera: Zygophlebiidae), NIGP163160; (B) Locustavidae (Orthoptera), NIGP162042; (C) Prochoristella (Mecoptera: Permochoristidae), NIGP162043; (D) Corixidae, NIGP162044; (E) Boreocixius (Hemiptera: Surijokocixiidae), NIGP162045; (F) Dunstaniidae (Hemiptera), NIGP162046; (G) Aphidoidea (Hemiptera), NIGP162047; (H) Chauliodites (Grylloblattida: Chaulioditidae), NIGP162048; (I) Cicadocorinae (Hemiptera: Progonocimicidae), NIGP162049; (J) Ichnogenus Folindusia (Trichoptera), NIGP162050; (K) Ichnogenus Terrindusia (Trichoptera), NIGP162051; (L) Myxophaga (Coleoptera), NIGP162052; (M) Elateriformia (Coleoptera), NIGP162053; (N) Dytiscoidea (Coleoptera), NIGP162054; (O) Cicadocorinae (Hemiptera: Progonocimicidae), NIGP162055. Scale bars, 5 mm (white), and 1 mm (black).
Most of Triassic beetles are preserved as isolated elytra (33–36). However, there are diverse beetles with bodies in the Tongchuan entomofauna, including the earliest putative Myxophaga (characterized by an elongate body with short elytra and widely separated mesocoxae and metacoxae; Fig. 3L), Elateriformia (characterized by a robust body with a distinct prosternal intercoxal process and produced hind pronotal angles; Fig. 2M), and Dytiscoidea (characterized by a streamlined body with two eyes, smooth elytra, and second abdominal sternite divided by metacoxae; Fig. 2N). These fossils, therefore, are potential key calibration points for the molecular phylogenetic analysis of beetle phylogeny.
Another interesting discovery is the earliest caddisfly cases (Trichoptera) in both entomofaunas (Fig. 3, J and K). Larval caddisflies construct cases using sand grains, shells, fish scales, coprolites, or vegetation cemented with the silk they secrete (23). The order Trichoptera is the sister to the Lepidoptera, of which the earliest record was near the Triassic-Jurassic boundary (37). The true Trichoptera was thought to have diverged from Amphiesmenoptera in the earliest Jurassic, and the earliest previously identified caddisfly case fossil was from the Early Jurassic of Siberia (Fig. 4) (23, 38). The Permian and Triassic “trichopteran” most probably belongs to the stem group of Amphiesmenoptera (39). The Permian marine “caddisfly” cases (40) were probably incorrectly identified since the caddisfly larva cannot build these cases with short, thick silk bundles (maximum, 1 mm diameter) arranged in an overlapping manner and not transversely circular. These marine cases were probably constructed by worms. The ichnogenus Folindusia (narrow-long, straight case built mainly of densely packed plant fragments, with some transverse arranged; Fig. 3J) from the Tongchuan entomofauna and the ichnogenus Terrendusia (narrow-long, straight case built of small densely and irregularly packed sand grains; Fig. 3K) from the Karamay entomofauna suggest an unexpected Middle Triassic origin of the construction behavior, much earlier than previously proposed from molecular studies (41, 42).
Fig. 4. Triassic terrestrial biodiversifications.
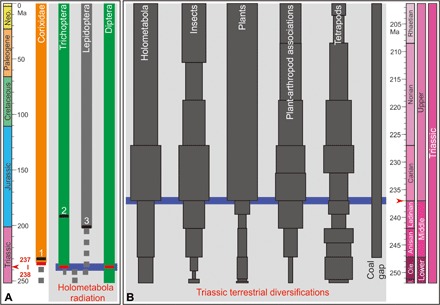
(A) Middle Triassic Holometabola radiation and (B) Triassic terrestrial diversifications. The previously earliest records of (1) Corixidae (45), (2) Trichoptera (38), and (3) Lepidoptera (37) are marked by black rectangular bars, and the new records reported in the present study are marked by red rectangular bars. The Triassic terrestrial diversifications are based on the data of holometabola [family richness; (63)], insects [family richness; (14)], plants [generic richness; (9, 64)], plant-arthropod associations [relative richness; (15)], tetrapods [generic richness; (65)], and coal gap (66). I., Induan; Ole., Olenekian; Neo., Neogene.
Middle-Late Triassic insect radiation
The Holometabola are the most diverse insects in the Tongchuan and Karamay entomofaunas, which are represented by diverse beetles and scorpionflies. Nowadays, holometabolous insects have the highest number of species of any clade and greatly contribute to the animal species biodiversity (43). Although they are already known from the Early Carboniferous (41, 43) and diversified during the Early-Middle Triassic (34), Holometabola were thought to have become dominant in the global entomofauna starting in the mid-Mesozoic (24, 44). Thus, its high diversity and abundance in Tongchuan and Karamay are relatively unexpected, revealing a radiation of Holometabola during the Middle Triassic, in correspondence to the results found from the Triassic of the Vosges (France) (34).
Aquatic insects also experienced substantial expansions in the Middle Triassic. Some key aquatic holometabolous clades, now comprising the bulk of modern freshwater biodiversity, can also be tracked back to the Middle Triassic origins, such as caddisflies and aquatic beetles (including Myxophaga and Dytiscoidea) found in both entomofaunas. In addition, water boatmen (characterized by fully sclerotized forewings with a distinct claval suture and no veins) are abundant in the Karamay entomofauna, and they are the earliest record of aquatic bugs (Fig. 3D). This finding is slightly earlier than those found from the Upper Triassic Huangshanjie Formation of Xinjiang (see the Supplementary Materials) (45) and the Cow Branch Formation of United States (22) and is earlier than the divergence times predicted on the basis of molecular studies (estimated to have occurred in the earliest Jurassic) (Fig. 4) (46). Together with true flies from other Early and Middle Triassic entomofaunas, these aquatic insects developed new herbivore and carnivore guilds that persist to the present day (47). The diversification of aquatic insects (mainly belonging to holometabolous insects) is thought to be part of the Mesozoic Lacustrine Revolution, which was dated as “Middle Mesozoic” (25, 48). However, our results suggest that this diversification has already begun by the Middle Triassic, thus providing new insights into the early evolution of freshwater ecosystems.
In summary, our findings confirm that holometabolous and aquatic insects experienced a radiation event in the Middle Triassic. Compared to other insects, Holometabola (including some aquatic insects) were probably more resilient to Early Triassic environmental disturbances because their development would have allowed greater buffering from environmental variability [analogous to modern species with a protective pupal stage, faster development, higher population sizes, and reduced intraspecific competition between the adult and offspring (49)]. After the Early Triassic, various plants (including aquatic plants) started to appear during the Middle Triassic and spread later (1), probably further contributing to the radiation of holometabolous and aquatic insects (Fig. 4).
Terrestrial Ladinian-Carnian boundary
The marine GSSP (Global Boundary Stratotype Section and Point) of the Carnian Stage is defined by the first appearance of the ammonoid Daxatina canadensis in the Prati Di Stuores/Stuores Wiesen section in the Southern Alps, Northeast Italy (50). Chemical abrasion–thermal ionization mass spectrometry (CA-TIMS) gave a precise age of 237.77 ± 0.052 Ma ago for the ash bed from the Alpe di Siusi/Seiser Alm area, 24 km west of the GSSP. The ash bed is lower than the GSSP point, and the age of the boundary is estimated on the basis of sediment accumulation (50). The Ladinian-Carnian boundary, ~237 Ma ago, is also supported by previous dating work on several strata correlated with the GSSP (51–53). The insect-rich Tongchuan terrestrial biota dated to ca. 238 to 237 Ma ago (Fig. 2) is in greement with the marine Ladinian-Carnian boundary. It should be noted that the marine and land strata can be correlated not only by isotopic ages but also by similar spore-pollen compositions. The GSSP is indicated by typical Carnian spore and pollen species Vallasporites ignacii and Patinasporites densus (50), which are absent in the Tongchuan Formation. In contrast, the appearance of some common insect elements in the Tongchuan, Madygen, and Australian entomofaunas indicates the close relationship among these Middle-Late Triassic terrestrial biotas. Therefore, our new age can provide a calibration point for marine and terrestrial correlations near the Ladinian-Carnian boundary and for the correlation of contemporaneous biotas.
MATERIALS AND METHODS
Insect fossils
The insect fossils were prepared using sharp pins. Photographs were taken using a Sony α7 camera and a Zeiss Discovery V16 microscope system with specimens moistened in 95% alcohol or dry. The figures were prepared with CorelDRAW X7 and Adobe Photoshop CS6.
U-Pb geochronology
The tuffaceous sandstone samples were crushed and separated to isolate the 80- to 200-μm grain size fraction (54). A total of 200 inclusion-free zircon grains from each sample were then picked under a binocular microscope and mounted in epoxy resin. Hardened mounts were polished to expose zircon grain midsections at about two-thirds to one-half of their widths. CL imaging documented grain morphologies and internal structure for in situ analysis. U-Pb isotopic data from zircons were obtained at the Department of Earth Sciences, The University of Hong Kong, using a Nu Instruments MC-ICP-MS with a Resonetics RESOlution M-50-HR Excimer Laser Ablation System. The analyses used a beam diameter of 30 μm, a repetition rate of 4 Hz, and an energy density of 5 J/cm2 on sample surface. Average ablation time was ca. 40 s, and pit depths reached about 30 μm. The Harvard reference zircon 91500 (1065.4 ± 0.3 Ma ago) (55) and GJ-1 zircon (609 Ma ago) (56) were used for preliminary calibration and second reference, respectively. Detailed operational procedures are found by Xia et al. (57). We used ICPMSDataCal (58) to process the offline signal selection, quantitative calibration, and time-drift correction. We used a function given by Anderson (59) to correct for common Pb in Microsoft Excel. Isoplot v. 3.0 (60) was used to construct concordia diagrams and probability density plots. Within the overall detrital age distribution, we cited 206Pb/238U ages for zircon grains younger than 1000 Ma ago and 207Pb/206Pb ages for older grains. Here, 100 zircon grains were randomly selected from each sample, so the results were expected to reflect the characteristics of the age populations. Ages with a discordance degree of >10% were excluded from the analysis (61).
Supplementary Material
Acknowledgments
We are grateful to A. Rasnitsyn, A. Ponomarenko, A. Bashkuev, J. Szwedo, A. Ślipiński, E. Yan, C. Cai, and O. Béthoux for helpful discussions and X. Zhou and two anonymous reviewers for careful comments that improved this manuscript. Funding: This research was supported by the HKU (The University of Hong Kong) Seed Funding Program for Basic Research (201411159057), the National Natural Science Foundation of China (41572010, 41622201, 41688103, and 41730317), and the Chinese Academy of Sciences (XDB26000000). This work is a contribution to UNESCO-IUGS IGCP (United Nations Educational, Scientific and Cultural Organization–International Union of Geological Sciences International Geological Correlation Programme) Project 632. This is a Leverhulme Emeritus Fellowship contribution for E.A.J. Author contributions: D.Z., S.-C.C., and B.W. designed the research project and wrote the manuscript. D.Z., H.W., Y.F., G.X., H.Z., and B.W. conducted the fieldwork. D.Z., S.-C.C., J.W., and C.F. conducted the geochronological analysis. D.Z., H.W., Y.F., E.A.J., H.Z., and B.W. identified the fossils. All authors discussed and approved the final manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. Correspondence and requests should be addressed to S.-C.C. and B.W.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaat1380/DC1
Supplementary Materials and Methods
Fig. S1. Photographs showing outcrops bearing Tongchuan and Karamay entomofaunas.
Table S1. Insect list of the Tongchuan entomofauna.
Table S2. U-Pb analytical results for samples from the Tongchuan outcrop.
REFERENCES AND NOTES
- 1.Benton M. J., Newell A. J., Impacts of global warming on Permo-Triassic terrestrial ecosystems. Gondwana Res. 25, 1308–1337 (2014). [Google Scholar]
- 2.Benton M. J., The Triassic. Curr. Biol. 26, R1214–R1218 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Trotter J. A., Williams I. S., Nicora A., Mazza M., Rigo M., Long-term cycles of Triassic climate change: A new δ18O record from conodont apatite. Earth Planet. Sci. Lett. 415, 165–174 (2015). [Google Scholar]
- 4.Benton M. J., The origins of modern biodiversity on land. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 3667–3679 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser N. C., Sues H.-D., The beginning of the ‘Age of Dinosaurs’: A brief overview of terrestrial biotic changes during the Triassic. Earth Env. Sci. Trans. R. Soc. 101, 189–200 (2011). [Google Scholar]
- 6.Marsicano C. A., Irmis R. B., Mancuso A. C., Mundil R., Chemale F., The precise temporal calibration of dinosaur origins. Proc. Natl. Acad. Sci. U.S.A. 113, 509–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Z.-X., Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Looy C. V., Brugman W. A., Dilcher D. L., Visscher H., The delayed resurgence of equatorial forests after the Permian–Triassic ecologic crisis. Proc. Natl. Acad. Sci. U.S.A. 96, 13857–13862 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grauvogel-Stamm L., Ash S. R., Recovery of the Triassic land flora from the end-Permian life crisis. C. R. Palevol 4, 593–608 (2005). [Google Scholar]
- 10.Żyła D., Wegierek P., Owocki K., Niedźwiedzki G., Insects and crustaceans from the latest Early–early Middle Triassic of Poland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 371, 136–144 (2013). [Google Scholar]
- 11.Haig D. W., Martin S. K., Mory A. J., McLoughlin S., Backhouse J., Berrell R. W., Kear B. P., Hall R., Foster C. B., Shi G. R., Bevan J. C., Early Triassic (early Olenekian) life in the interior of East Gondwana: Mixed marine–terrestrial biota from the Kockatea Shale, Western Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 417, 511–533 (2015). [Google Scholar]
- 12.Ponomarenko A. G., Insects during the time around the Permian—Triassic crisis. Paleontol. J. 50, 174–186 (2016). [Google Scholar]
- 13.Labandeira C. C., The fossil record of insect extinction: New approaches and future directions. Am. Entomol. 51, 14–29 (2005). [Google Scholar]
- 14.Clapham M. E., Karr J. A., Nicholson D. B., Ross A. J., Mayhew P. J., Ancient origin of high taxonomic richness among insects. Proc. Biol. Sci. 283, 20152476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labandeira C. C., The four phases of plant–arthropod associations in deep time. Geol. Acta 4, 409–438 (2006). [Google Scholar]
- 16.Labandeira C. C., Currano E. D., The fossil record of plant-insect dynamics. Annu. Rev. Earth Planet. Sci. 41, 287–311 (2013). [Google Scholar]
- 17.Wappler T., Kustatscher E., Dellantonio E., Plant-insect interactions from Middle Triassic (late Ladinian) of Monte Agnello (Dolomites, N-Italy)—Initial pattern and response to abiotic environmental perturbations. PeerJ 3, e921 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labandeira C. C., Kustatscher E., Wappler T., Floral assemblages and patterns of insect herbivory during the Permian to Triassic of Northeastern Italy. PLOS ONE 11, e0165205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Béthoux O., Papier F., Nel A., The Triassic radiation of the entomofauna. C. R. Palevol 4, 609–621 (2005). [Google Scholar]
- 20.Kustatscher E., Franz M., Heunisch C., Reich M., Wappler T., Floodplain habitats of braided river systems: Depositional environment, flora and fauna of the Solling Formation (Buntsandstein, Lower Triassic) from Bremke and Fürstenberg (Germany). Palaeobiodivers. Palaeoenviron. 94, 237–270 (2014). [Google Scholar]
- 21.van Eldijk T., Goris G., Haarhuis A., Lankamp J., Winkelhorst H., Reumer J., Nel A., Wappler T., New fossil insects from the Anisian (Lower to Middle Muschelkalk) from the Central European Basin (Germany and The Netherlands). PalZ 91, 185–194 (2017). [Google Scholar]
- 22.Fraser N. C., Grimaldi D., Olsen P. E., Axsmith B., A Triassic Lagerstätte from eastern North America. Nature 380, 615–619 (1996). [Google Scholar]
- 23.D. Grimaldi, M. S. Engel, Evolution of the Insects (Cambridge Univ. Press, 2005). [Google Scholar]
- 24.Peters R., Meusemann K., Petersen M., Mayer C., Wilbrandt J., Ziesmann T., Donath A., Kjer K. M., Aspöck U., Aspöck H., Aberer A., Stamatakis A., Friedrich F., Hünefeld F., Niehuis O., Beutel R. G., Misof B., The evolutionary history of holometabolous insects inferred from transcriptome-based phylogeny and comprehensive morphological data. BMC Evol. Biol. 14, 52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A. S. Cohen, Paleolimnology: The History and Evolution of Lake Systems (Oxford Univ. Press, 2003). [Google Scholar]
- 26.Cohen K. M., Finney S. C., Gibbard P. L., Fan J.-X., The ICS international chronostratigraphic chart. Episodes 36, 199–204 (2013). [Google Scholar]
- 27.Andersen T., Detrital zircons as tracers of sedimentary provenance: Limiting conditions from statistics and numerical simulation. Chem. Geol. 216, 249–270 (2005). [Google Scholar]
- 28.Dickinson W. R., Gehrels G. E., Use of U–Pb ages of detrital zircons to infer maximum depositional ages of strata: A test against a Colorado Plateau Mesozoic database. Earth Planet. Sci. Lett. 288, 115–125 (2009). [Google Scholar]
- 29.Gehrels G., Detrital zircon U-Pb geochronology applied to tectonics. Annu. Rev. Earth Planet. Sci. 42, 127–149 (2014). [Google Scholar]
- 30.Zhang H., Peng P., Zhang W., Zircon U-Pb ages and Hf isotope characterization and their geological significance in the Chang 7 tuff of the Yanchang Formation in the Ordos Basin. Acta Petrol. Sin. 30, 565–575 (2014). [Google Scholar]
- 31.Shcherbakov D. E., Insect recovery after the Permian/Triassic crisis. Alavesia 2, 125–131 (2008). [Google Scholar]
- 32.Szwedo J., Lapeyrie J., Nel A., Rooting down the aphid’s tree—The oldest record of the Aphidomorpha lineage from the Palaeozoic (Insecta: Hemiptera). Syst. Entomol. 40, 207–213 (2015). [Google Scholar]
- 33.A. P. Rasnitsyn, D. L. J. Quicke, History of Insects (Kluwer Academic Publishers, 2002). [Google Scholar]
- 34.Papier F., Nel A., Grauvogel-Stamm L., Gall J.-C., La diversité des Coleoptera (Insecta) du Trias dans le nord-est de la France. Geophys. J. Roy. Astron. Soc. 27, 181–199 (2005). [Google Scholar]
- 35.Martins-Neto R. G., Gallego O. F., Tassi L. V., The Triassic coleopteran fauna of southern. J. Geophys. Res. 7, 1–18 (2011). [Google Scholar]
- 36.Meller B., Ponomarenko A. G., Vassilenko D. V., Fischer T. C., Aschauer B., First beetle elytra, abdomen (Coleoptera) and a mine trace from Lunz (Carnian, Late Triassic, Lunz-am-See, Austria) and their taphonomical and evolutionary aspects. Palaeontology 54, 97–110 (2011). [Google Scholar]
- 37.van Eldijk T. J. B., Wappler T., Strother P. K., van der Weijst C. M. H., Rajaei H., Visscher H., van de Schootbrugge B., A Triassic-Jurassic window into the evolution of Lepidoptera. Sci. Adv. 4, e1701568 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.I. D. Sukatsheva, in Jurassic Caddisflies of Southern Siberia, A. P. Rasnitsyn, Ed. (Trudy Paleontologischeskogo Instituta Akademii Nauk, SSSR, 1985), pp. 115–119. [Google Scholar]
- 39.G. Bechly, in The Crato Fossil Beds of Brazil: Window into an Ancient World, D. M. Martill, G. Bechly, R. F. Loveridge, Eds. (Cambridge Univ. Press, 2007), pp. 387–393. [Google Scholar]
- 40.Mouro L. D., Zatoń M., Fernandes A. C. S., Waichel B. L., Larval cases of caddisfly (Insecta: Trichoptera) affinity in Early Permian marine environments of Gondwana. Sci. Rep. 6, 19215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misof B., Liu S., Meusemann K., Peters R. S., Donath A., Mayer C., Frandsen P. B., Ware J., Flouri T., Beutel R. G., Niehuis O., Petersen M., Izquierdo-Carrasco F., Wappler T., Rust J., Aberer A. J., Aspöck U., Aspöck H., Bartel D., Blanke A., Berger S., Böhm A., Buckley T. R., Calcott B., Chen J., Friedrich F., Fukui M., Fujita M., Greve C., Grobe P., Gu S., Huang Y., Jermiin L. S., Kawahara A. Y., Krogmann L., Kubiak M., Lanfear R., Letsch H., Li Y., Li Z., Li J., Lu H., Machida R., Mashimo Y., Kapli P., McKenna D. D., Meng G., Nakagaki Y., Luis Navarrete-Heredia J., Ott M., Ou Y., Pass G., Podsiadlowski L., Pohl H., von Reumont B. M., Schütte K., Sekiya K., Shimizu S., Slipinski A., Stamatakis A., Song W., Su X., Szucsich N. U., Tan M., Tan X., Tang M., Tang J., Timelthaler G., Tomizuka S., Trautwein M., Tong X., Uchifune T., Walzl M. G., Wiegmann B. M., Wilbrandt J., Wipfler B., Wong T. K. F., Wu Q., Wu G., Xie Y., Yang S., Yang Q., Yeates D. K., Yoshizawa K., Zhang Q., Zhang R., Zhang W., Zhang Y., Zhao J., Zhou C., Zhou L., Ziesmann T., Zou S., Li Y., Xu X., Zhang Y., Yang H., Wang J., Wang J., Kjer K. M., Zhou X., Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Malm T., Johanson K. A., Wahlberg N., The evolutionary history of Trichoptera (Insecta): A case of successful adaptation to life in freshwater. Syst. Entomol. 38, 459–473 (2013). [Google Scholar]
- 43.Nel A., Roques P., Nel P., Prokin A. A., Bourgoin T., Prokop J., Szwedo J., Azar D., Desutter-Grandcolas L., Wappler T., Garrouste R., Coty D., Huang D., Engel M. S., Kirejtshuk A. G., The earliest known holometabolous insects. Nature 503, 257–261 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Tapanila L., Roberts E. M., The earliest evidence of holometabolan insect pupation in conifer wood. PLOS ONE 7, e31668 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Q., Triassic insect fauna from Toksun, Xinjiang. Acta Palaeontol. Sin. 31, 314–335 (1992). [Google Scholar]
- 46.Wang Y.-H., Cui Y., Rédei D., Baňař P., Xie Q., Štys P., Damgaard J., Chen P.-P., Yi W.-B., Wang Y., Dang K., Li C.-r., Bu W.-j., Phylogenetic divergences of the true bugs (Insecta: Hemiptera: Heteroptera), with emphasis on the aquatic lineages: The last piece of the aquatic insect jigsaw originated in the Late Permian/Early Triassic. Cladistics 32, 390–405 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Dijkstra K.-D. B., Monaghan M. T., Pauls S. U., Freshwater biodiversity and aquatic insect diversification. Annu. Rev. Entomol. 59, 143–163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponomarenko A. G., Evolution of continental aquatic ecosystems. Paleontol. J. 30, 705–709 (1996). [Google Scholar]
- 49.Nicholson D. B., Ross A. J., Mayhew P. J., Fossil evidence for key innovations in the evolution of insect diversity. Proc. Biol. Sci. 281, 20141823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mietto P., Manfrin S., Preto N., Rigo M., Roghi G., Furin S., Gianolla P., Posenato R., Muttoni G., Nicora A., Buratti N., Crilli S., Spitl C., Ramezani J., Bowring S. A., The Global Boundary Stratotype Section and Point (GSSP) of the Carnian Stage (Late Triassic) at Prati Di Stuores/Stuores Wiesen Section (Southern Alps, NE Italy). Episodes 35, 414–430 (2012). [Google Scholar]
- 51.Mundil R., Brack P., Meier M., Rieber H., Oberli F., High resolution U-Pb dating of Middle Triassic volcaniclastics: Time-scale calibration and verification of tuning parameters for carbonate sedimentation. Earth Planet. Sci. Lett. 141, 137–151 (1996). [Google Scholar]
- 52.Brack P., Rieber H., Nicora A., Mundil R., The Global boundary Stratotype Section and Point (GSSP) of the Ladinian Stage (Middle Triassic) at Bagolino (Southern Alps, Northern Italy) and its implications for the Triassic time scale. Episodes 28, 233–244 (2005). [Google Scholar]
- 53.Brühwiler T., Hochuli P. A., Mundil R., Schatz W., Brack P., Bio- and chronostratigraphy of the Middle Triassic Reifling Formation of the westernmost Northern Calcareous Alps. Swiss J. Geosci. 100, 443–455 (2007). [Google Scholar]
- 54.Wang J., Chang S.-C., Wang K.-L., Lu H.-B., Zhang H.-C., Geochronology and geochemistry of the Early Cretaceous igneous units from the central Sulu orogenic belt: Evidence for crustral delamination during a shift in the regional tectonic regime. J. Asian Earth Sci. 112, 49–59 (2015). [Google Scholar]
- 55.Wiedenbeck M., Allé P., Corfu F., Griffin W. L., Meier M., Oberli F., Quadt A., Roddick J. C., Spiegel W., Three natural zircon standards for U–Th–Pb, Lu–Hf, trace element and REE analyses. Geostand. Geoanal. Res. 19, 1–23 (1995). [Google Scholar]
- 56.Jackson S. E., Pearson N. J., Griffin W. L., Belousova E. A., The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 211, 47–69 (2004). [Google Scholar]
- 57.Xia X., Sun M., Geng H., Sun Y., Wang Y., Zhao G., Quasi-simultaneous determination of U–Pb and Hf isotope compositions of zircon by excimer laser-ablation multiple-collector ICPMS. J. Anal. At. Spectrom. 26, 1868–1871 (2011). [Google Scholar]
- 58.Liu Y., Gao S., Hu Z., Gao C., Zong K., Wang D., Continental and oceanic crust recycling-induced melt–peridotite interactions in the trans-north China orogen: U–Pb dating, Hf isotopes and trace elements in zircons from mantle xenoliths. J. Petrol. 51, 537–571 (2010). [Google Scholar]
- 59.Anderson T., Correction of common lead in U–Pb analyses that do not report 204Pb. Chem. Geol. 192, 59–79 (2002). [Google Scholar]
- 60.K. R. Ludwig, Isoplot v. 3.0: A Geochronological Toolkit for Microsoft Excel: Special Publication, No. 4 (Berkeley Geochronology Center, 2003).
- 61.Prokopiev A. V., Toro J., Miller E. L., Gehrels G. E., The paleo-Lena River–200 m.y. of transcontinental zircon transport in Siberia. Geology 36, 699–702 (2008). [Google Scholar]
- 62.Golonka J., Late Triassic and Early Jurassic palaeogeography of the world. Palaeogeogr. Palaeoclimatol. Palaeoecol. 244, 297–307 (2007). [Google Scholar]
- 63.Nicholson D. B., Mayhew P. J., Ross A. J., Changes to the fossil record of insects through fifteen years of discovery. PLOS ONE 10, e0128554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong C., Wang Q., Permian–Triassic land-plant diversity in South China: Was there a mass extinction at the Permian/Triassic boundary? Paleobiology 37, 157–167 (2011). [Google Scholar]
- 65.Benton M. J., Ruta M., Dunhill A. M., Sakamoto M., The first half of tetrapod evolution, sampling proxies, and fossil record quality. Palaeogeogr. Palaeoclimatol. Palaeoecol. 372, 18–41 (2013). [Google Scholar]
- 66.Retallack G. J., Veevers J. J., Morante R., Global coal gap between Permian-Triassic extinction and Middle Triassic recovery of peat-forming plants. Geol. Soc. Am. Bull. 108, 195–207 (1996). [Google Scholar]
- 67.Institute of Geology, Chinese Academy of Geological Sciences, in Mesozoic Stratigraphy and Palaeontology of the Shanganning Basin (Beijing: Geology Press, 1980). [Google Scholar]
- 68.Zhang W., Yang H., Xia X., Xie L., Xie G., Triassic chrysophyte cyst fossils discovered in the Ordos Basin, China. Geology 44, 1031–1034 (2016). [Google Scholar]
- 69.Zheng D. R., Nel A., Wang B., Jarzembowski E. A., Chang S.-C., Zhang H. C., The first Triassic “Protodonatan” (Zygophlebiidae) from China: Stratigraphical implications. Geol. Mag. 154, 169–174 (2017). [Google Scholar]
- 70.Guo X., Hong Y., New genus and species of Permochoristidae Tillyard (Insecta, Mecoptera) from the Middle Triassic Tongchuan Formation, Shaanxi Province, China. Acta Zootaxonomica Sin. 28, 712–715 (2003). [Google Scholar]
- 71.Hong Y., First discovery of mid-Triassic order Miomoptera (Insecta) in China. Geol. Bull. China 28, 11–15 (2009). [Google Scholar]
- 72.Luo Z., Shi T., Tang P., Huang P., Zheng D., Wan M., Wang X., Yin Y., Restudy of the age of the Karamay Formation in the northwestern margin of the Junggar basin. Xinjiang Petrol. Geol. 36, 668–681 (2015). [Google Scholar]
- 73.Luo Z.-J., Wang R., Zhao J.-H., A L.-Y., Late Permian-Middle Jurassic megaspores assemblages in the northwest area, Junggar basin. Xinjiang Geol. 25, 243–247 (2007). [Google Scholar]
- 74.Qiu X.-W., Liu C.-Y., Mao G.-Z., Wu B.-L., Petrological-geochemical characteristics of volcanic ash sediments in the Yanchang Formation in the Ordos Basin (in Chinese). Earth Sci. J. China Univ. Geosci. 36, 139–150 (2011). [Google Scholar]
- 75.Liu J., Li L., Li X., Shrimp U-Pb zircon dating of the Triassic Ermaying and Tongchuan formations in Shanxi, China and its stratigraphic implications. Vertebr. Palasiat. 51, 162–168 (2013). [Google Scholar]
- 76.Wang D., Xin B., Yang H., Fu J., Yao J., Zhang Y., Zircon SHRIMP U-Pb age and geological implications of the tuff at the bottom of the Chang-7 Member of the Yanchang Formation in the Ordos Basin. Sci. China Earth Sci. 57, 2966–2977 (2014). [Google Scholar]
- 77.Deng X., Li W., Liu X., Pang J., Liu X., Discussion on the stratigraphic boundary between the Middle Triassic and Upper Triassic. Acta Geol. Sin. 83, 1089–1096 (2009). [Google Scholar]
- 78.Jiang D., Wang Y., Wei J., Palynoflora and its environmental significance in the Late Triassic in Tongchuan, Shaanxi Province. J. Palaeogeogr. 8, 23–33 (2006). [Google Scholar]
- 79.Wang Y., Jiang D., Xie X., Late Triassic palynoflora and its environmental significance at Tuweihe, Shaanxi. Acta Sedimentol. Sin. 21, 434–440 (2003). [Google Scholar]
- 80.Wu X., Liu J., Li J., The anatomy of the first archosauriform (Diapsida) from the terrestrial Upper Triassic of China. Vertebr. Palasiat. 39, 251–265 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/9/eaat1380/DC1
Supplementary Materials and Methods
Fig. S1. Photographs showing outcrops bearing Tongchuan and Karamay entomofaunas.
Table S1. Insect list of the Tongchuan entomofauna.
Table S2. U-Pb analytical results for samples from the Tongchuan outcrop.


