Abstract
Daily oscillations of brain and body states are under complex temporal modulation by environmental light and the hypothalamic suprachiasmatic nucleus (SCN), the master circadian clock. To better understand mediators of differential temporal modulation, we characterize neuropeptide releasate profiles by non-selective capture of secreted neuropeptides in an optic nerve-horizontal SCN brain slice model. Releasates are collected following electrophysiological stimulation of the opticnerve/retinohypothalamic tract under conditions that alter the phase of SCN activity state. Secreted neuropeptides are identified by intact mass via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). We found time-of-day-specific suites of peptides released downstream of optic-nerve stimulation. Peptide release was modified differentially with respect to time-of-day by stimulus parameters and by inhibitors of glutamatergic or PACAPergic neurotransmission. The results suggest that SCN physiology is modulated by differential peptide release of both known and unexpected peptides that communicate time-of-day-specific photic signals via previously unreported neuropeptide signatures.
Keywords: circadian clock, mass spectrometry, neuropeptidomics, optic nerve, suprachiasmatic nucleus, SCN
INTRODUCTION
Biological processes, from tissue-specific gene ex pression to organismic behavior, display daily, near-24-h circadian rhythms1–3. The suprachiasmatic nucleus (SCN) of the ventral hypothalamus is the primary site that orchestrates circadian rhythms in mammals. Comprised of ~20,000 cells4–5 ,the mammalian SCN generates timekeeping signals and regulates timing homeostasis through integration of myriad neuronal and humoral signals, including information about environmental light. Time-of-day-spe- cific responses of the SCN then align internal circadian rhythms with the natural cycle of darkness and light.
Environmental light signals are communicated directly to the SCN by the retinohypothalamic tract (RHT). Axons of intrinsically photoreceptive retinal ganglion cells (ipRGCs) project to the SCN via the optic nerve (ON), transmitting light information directly from the retina to the hypothalamus. Within the SCN, light information is processed and integrated into time-of-day- specific adjustments of the SCN time-base6–8. Photosensitivity of ipRGCs adapts to background light levels, which leads to differential patterns of action potentials that communicate light information to the SCN9–11. Glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) are the chemical messengers of light information from synapses of the ipRGC onto SCN neurons12–14. Mechanisms of circadian sensitivity and responses of the SCN to these two neurochemicals are well studied 15. Light has different effects on circadian timing that depend on time-of-day15, and the RHT innervates only a subset of SCN neurons16. Consequently, some form(s) of inter-cellular communication must coordinate signaling and subsequent light-phase alignment among the ~20,000 cells of the SCN.
While mechanisms such as electrical coupling17–18 and inhibitory neurotransmission19 have some role in SCN intercellular communication, mounting evidence points to diffusible signaling as a key contributor to the SCN re sponses to photic signals20–21. Endogenous peptide expression among SCN neurons is heterogeneous4,22–24 and neuropeptides have been implicated in altering the phase of the circadian oscillation generated in the SCN, i.e., phase-shifting25–28. Gastrin-releasing peptide (GRP), vasoactive intestinal polypeptide (VIP), and little SAAS are expressed within retinorecipient SCN neurons and are involved in SCN feed-forward relay of phase shifting signals29–31. The roles of these peptides in intercellular SCN communication have been investigated using a combination of both genetic knockout and exogenous in vitro chemical stimulation,27,30–34 however confirmatory peptide release from the SCN downstream of light signal innervation has not been demonstrated.
Mass spectrometry-based approaches are effective for identifying suites of neuropeptides, the approach known as peptidomics35–37. Over the past decade we have performed extensive global characterization of the peptides contained within the SCN using these approaches35–37. However, such global measurements do not provide functional information. Thus, we have measured the neuropeptides released at the SCN following time-of-day-specific and light signal-associated stimulation conditions38. Unlike immunobased assays, our strategy utilizes non-selective capture and unbiased identification of neuropeptides secreted into the media. Released neuropeptides are collected at specified time intervals using C18-containing ZipTips™ positioned above the tissue region of interest, and captured releasates are eluted off-line and identified by intact masses measured by MALDI-TOF mass spectrometry38. Thus, heterogeneous release profiles of secreted peptides can be measured directly in spatial, temporal, and stimulation-dependent contexts without aprior knowledge of the secretomes15. Limitations in releasate abundances prevent direct MS/MS measurements on the releasate. Here we characterize peptides by comparison of intact mass values in releasate mass spectra to those reported from our high-resolution proteomic/peptidomic discovery studies using isolated subhypothalamic nuclei23–24, 39–40 These analytical chemistry protocols permit secreted peptide profiling and identification with spatial-, temporal-, and stimulation-dependent resolution15. We previously demonstrated endogenous peptide release could be detected from the SCN in ex vivo hypothalamic slice38. Building on this experimental paradigm, we here utilize a horizontal hypothalamic brain slice - retaining the SCN with ON and RHT innervation - to evaluate whether SCN peptide release is detected following different light signal-associated stimulus conditions that elicit circadian phase shifting of the SCN circadian clock.
RESULTS AND DISCUSSION
To determine the parameters of electrical stimulation of the ON that evoke phase-shifting of the SCN (Fig. 1, 2A), the CT of the subsequent peak of the mean spontaneous firing of the SCN neuronal population was compared with the CT under control conditions. In the unstimulated SCN, the spontaneous firing rate peaked during the mid-subjective daytime at CT 6.1 ± 0.28 (n = 5; Fig. 3A, O). When the ON was stimulated (5 V, 20 Hz, 1 ms pulse duration, Fig. 2A) at CT 6, the spontaneous firing rate peak advanced in phase compared to control (+2.85 ± 0.25 h, n = 3, p < 0.001; Fig. 3B, O). This phase-shift is comparable in direction and magnitude to that induced by exogenous PACAP applied to the SCN in a brain slice at the same CT41–42.
Figure 1.
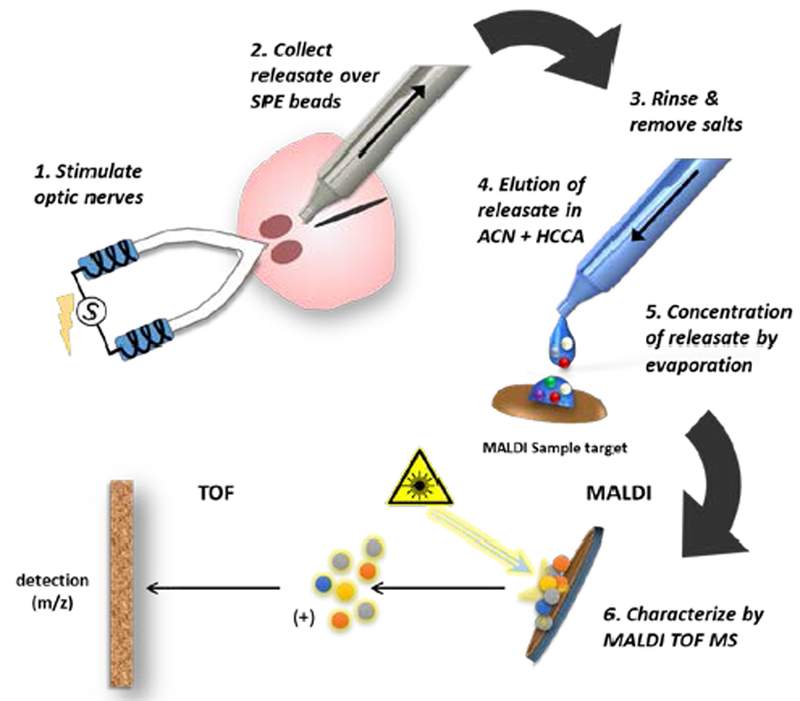
Schematic of the workflow for neuropeptide collection from the SCN brain slice and MS analysis. 1. The optic nerves (ON) attached to a horizontal SCN brain slice are stimulated in tandem by suction electrodes or the SCN is treated directly by droplet containing a chemical stimulus. 2. Releasate is aspirated through a micropipette containing solid-phase extraction beads (SPEs), which bind peptides based on their charge. 3. The beads are rinsed to remove salts and then samples (represented as small colored circles) are transferred to a MALDI target surface. 4. Bound analytes are eluted with acetonitrile (ACN) and addition of a cyano-4 hydroxycinnamic acid (HCCA) MALDI matrix solution. 5. As the acetonitrile evaporates, analytes are concentrated with MALDI matrix onto discrete hydrophobic regions within the HCCA crystals on the pre-treated target plate. 6. The sample is volatized and ionized by matrix-assisted laser desorption/ionization (MALDI). Following ionization, the analytes are subjected to a mass analyzer and detector for spectrophotometric analysis where their mass/charge ratio (m/z) is determined.
Figure 2.

Stimulus parameters applied to the ON to induce temporal phase shifting and peptide release in the SCN and outcomes. (A) Three different 5-min electrical stimulation paradigms were utilized: Orange, 1 V, 10 Hz, 0.2 ms pulse duration; Green, 5 V, 5 Hz, 1 ms pulse duration; Purple, 5 V, 20 Hz, 1 ms pulse duration. (B) These three stimulation parameters at distinct temporal windows caused different patterns of peptide release (■), and phase shifts of a delay of ~ 2 hr (φD), advance of ~2–3 hr (φA), or an advance of ~ 6 hr (φA+), respectively. N.R., no releasate measured.
Figure 3.
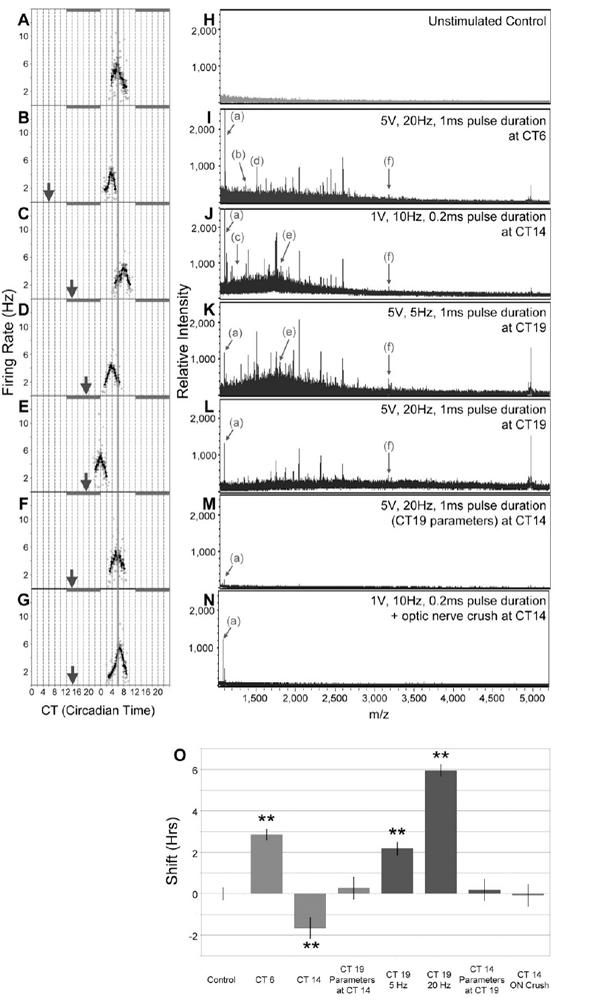
Stimulus- and time-of-day specific phase-shifts of SCN neuronal activity are accompanied by stimulus- and time-of-day-specific peptide release. Horizontal suprachiasmatic brain slices of the mediobasal hypothalamus and preserving the ONs, which contain retinal afferents to the SCN, were monitored in vitro. (A) The SCN displays a circadian rhythm in neuronal firing rate that peaks at mid-subjective day, CT 6. ONs were stimulated bilaterally via suction electrodes with specific current, frequency, and pulse duration parameters at circadian time points (arrowheads). During daytime (CT 6) and late subjective nighttime (CT 19), ON stimulation evokes phase advance of the neuronal activity rhythm (B, D, and E), while effective parameters at early subjective nighttime (CT 14) trigger phase delay of SCN firing rhythm (C). MALDI-TOF-MS analyses of releasate collected from the SCN exhibit time-of-day and stimulus-specific peptide release profiles (H-L). Some peptide peaks, such as arginine vasopressin (a) and galanin (f), are observed at every timepoint, while release of other peptides, such as neurokinin-B (b), somatostatin-28 (c), angiotensin (d), and little SAAS (e) is restricted to specific time-of-day and stimulus conditions. Effective parameters at CT 19 are ineffective in phase shift (F) and peptide release (M) at CT 14. Transectional ON crush prevents stimulus-evoked phase shifting (G)and peptide release (N). Phase-shifting responses to ON stimulation depend upon unique, time-of-day-effective stimulus parameters (O). **, p < 0.001, one-way ANOVA.
The efficacy of various stimulus parameters was explored with regard to degree of phase shift and potential signaling pathways.43 A 5 Hz stimulation applied at CT6 had no effect on the subsequent peak of circadian activity; however, a 20 Hz stimulation at CT 6 produced a phase advance that was blocked completely in the presence of a selective PACAP inhibitor, 10mm PACAP 6–38. PACAP 6–38 applied alone at CT6 had no effect on clock phase.
Previous research has demonstrated that exogenous glutamate, applied directly to SCN in vitro during the subjective nighttime, evokes phase delay when administered at CT 14 whereas at CT 19 causes phase advance12,26. We found that comparable time-dependent phase shifts could be induced in the horizontal SCN slice when ON stimulation is applied. Stimulation at CT 14 (1 V, 10 Hz, 0.2ms pulse duration, Fig. 2A) elicited a phase delay (−1.65 ± 0.5 h, n = 10, p < 0.001; Fig. 3C, O). During late subjective nighttime (CT 19), two ON stimulation parameters were effective in phase-advancing the rhythm of spontaneous SCN firing rate. Stimulating the ON at 5 V, 5 Hz, 1 ms pulse duration (Fig. 2A) triggered a glutamate-like phase shift in direction and magnitude (+2.18 ± 0.30 h, n = 6, p < 0.001; Fig. 3D, O). Increasing stimulus frequency to 20 Hz at CT 19 (Fig. 2A) generated a potentiated phase advance (+5.95 ± 0.29 h, n = 5, p < 0.001; Fig. 3E, O). Stimulation parameters that caused a phase advance at CT 19 were ineffective when applied at CT 14 (+0.27 ± 0.52 h, n= 3; Fig. 3F, O). Similarly, stimulus parameters effective CT 14 did not cause phase-shifting of the firing rate rhythm when applied at CT 19 (+0.18 ± 0.52 h, n = 3; Fig. 3O). This is consistent with the established time-dependency and directionality of the SCN to glutamate-induced phase-shifting during the nighttime 12. Physically crushing the ON prevented phase shifting via electrical stimulation (−0.07 ± 0.52 h, n =3; Fig. 3G, O). This demonstrates that the electrical stimuli act via neurotransmission, rather than through current spread, and their effects represent physiological correlates of time-of-day ipRGC photosensitivity9–11 and light-stimulated activation of the retinohypothalamic tract. The electrical stimuli, therefore, evoke acute light-like responses and subsequent phase shifting in the SCN12.
Next, we coupled electrical stimulation of the ON with ZipTip capture above the SCN region and subsequent MALDI TOF MS analysis to characterize neuropeptide release profiles following ON-stimulation paradigms that shift the phase of SCN activity. ON stimulation parameters that were effective in phase-shifting at CT 6 (Fig. 3I), CT 14 (Fig. 3J), and CT 19 (5 Hz and 20 Hz, Fig. 3K and L, respectively) were correlated with distinct peptide release profiles in a stimulus- and time-of-day-specific manner (n ≥ 3 for all releasate sample conditions, Fig. 3B). Peptide-release profiles obtained from tissue subjected to stimulation parameters ineffective at phase shifting or ON crush (Fig. 3M and N) were comparable to the pre-stimulation releasate sample control (Fig. 3H). Previously, we have performed a number of studies on the peptides contained within SCN tissue using a variety of high resolution tandem MS approaches; the amount of peptide released from the brain slice in response to the electrical stimulation precludes the use of tandem MS 23–24’ 39–40. Here we combine our lists of previously identified SCN peptides with release profiles to assign putative masses to known SCN peptides (Table 1). Despite our detailed prior studies, we detect a number of m/z values that are not in our databases. This may be due to differences in the ionization method used. Our previous assignments were based on electrospray ionization MS whereas the current studies required the superior sensitivity of MALDI-TOF MS, which has high ion transmission rate and lowest residence time in the vacuum. Also, the previous studies analyzed tissue punches of SCN from coronal brain slices compared with the present study which analyzed SCN with attached optic nerves, wherein distinct peptides could be contained within the RHT and/or horizontally sliced SCN. These unassigned masses are listed in Supplemental Information (SI, Table 1).
Table 1. Peptides detected directly from stimulated releasate of the suprachiasmatic nucleus.
| Putative Peptide (Prohormone) Name | Observed m/z (MH+) | Theoretical m/z (MH+) | Ref | RHT Stimulus-Triggered Releasate Conditions | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Stim | CT6 | CT14 | CT19 (5Hz) | CT19 (20Hz) | ||||
| Angiotensin I (Angiotensinogen) | 1296.62 | 1296.69 | 38 | • | ||||
| Little SAAS (Pro-SAAS) | 1784.97 | 1784.98 | 23, 38–39 | • | • | |||
| Big LEN (Pro-SAAS) | 1745.95 | 1745.97 | 38 | • | ||||
| PEN (Pro-SAAS) | 2301.25 | 2301.24 | 38–39 | • | • | • | • | |
| Cerebellin, aa57–71 (Cerebellin-1) | 1495.75 | 1495.79 | 23 | ‡ | • | • | • | |
| Galanin (Galanin) | 3163.59 | 3163.58 | 23, 38 | • | • | • | • | |
| P-endorphin (POMC) | 3463.80 | 3463.86 | 61 | • | ||||
| Melanotropin α (POMC) | 1622.78 | 1622.79 | 38, 40 | • | ||||
| Morphogenetic Neuropeptide (HA) | 1141.68 | 1141.67 | 61 | • | ||||
| Neurokinin-B | 1210.56 | 1210.54 | 38 | • | ||||
| Pro-enkephalin A, aa219–229 (PENK) | 1466.64 | 1466.65 | 23, 38, 40 | • | ||||
| ProSomatostatin 89–100 | 1244.58 | 1244.57 | 38 | • | ||||
| Somatostatin 14 | 1637.73 | 1637.71(ss) | 38 | • | ||||
| Substance P (Protachykinin-1 (PPT)) | 1347.73 | 1347.74 | 23, 38, 40 | • | ||||
| Thymosin β−4 (Thymosin β−4) | 4961.50 | 4961.49(a) | 38 | ‡ | • | • | • | • |
| Arginine-Vasopressin (AVP-NPII) | 1084.46 | 1084.46(ss) | 38, 40 | ‡ | • | • | • | • |
| Vasopressin-Neurophysin2-copeptin, aa151–168 (AVP-NPII) | 1948.00 | 1948.01 | 23 | • | ||||
| Vasopressin-Neurophysin2-copeptin, aa154–168 (AVP-NPII) | 1607.80 | 1607.80 | 23 | • | ||||
| MBP, aa2–18 (MBP) | 2028.10 | 2028.07 (a) | 39 | • | • | |||
| Secretogranin 2, aa598–612 [Secretogranin 2] | 1785.81 | 1785.85 | 23 | • | • | |||
, Corresponding peaks identified in < 50% of the samples taken for the particular Releasate Condition. (ss), mass w/disulfide bond; (a), mass w /acetylation; (p) mass w/pyro-glu PTM at Q
What are the functions of these peptides? A number of the molecular masses identified in stimulated releasate correspond to known, physiologically relevant peptides within the SCN. A salient example is arginine vasopression (AVP), which displays circadian oscillations in expression and release38, 44. A prominent peak in release occurs spontaneously in early daytime of SCN maintained in vitro45–46. AVP is expressed in the dorsomedial SCN6, 22, and acts as an output of the circadian clock, transmitting time-of-day information to other brain regions and the cerebral spinal fluid.
Whereas AVP acts as an output of the SCN, several of the identified peptides are components of intrinsic SCN circuits that process incoming signals. Our previous peptidomic studies identified little SAAS together with other peptides derived from the prohormone, ProSAAS26. Little SAAS-expressing neurons are the third most abundant peptidergic class in rat SCN26. They localize to the retinorecipient area, and ~50% are targets of light-stimulated cFOS-induction. Little SAAS neurons relay signals downstream of the photic/glutamatergic signaling from the eye to the SCN. The action of little SAAS in altering phase of the SCN is independent of pathways involving vasoactive intestinal peptide (VIP) and gastrin-releasing peptide (GRP) action 26, which also relay light signals within the SCN. Little SAAS partially colocalizes with VIP- and GRP-expressing neurons. The functions of other ProSAAS-derived peptides, such as Big LEN and PEN, within the SCN remain unknown.
Many of the multitude of peptides identified within the SCN have established functions in other brain regions, but their contributions in the SCN are less well understood. Several of these include the following. Angiotensin II (ANGII), derived by the action of angiotensin-converting enzyme (ACE), along with its cognate receptor, AT1, are expressed in the SCN. ANGII-AT1 signaling is involved in the depolarization of SCN neurons47. Prepropeptide mRNAs encoding opiom elanocortin (POMC) and secretogranin have been localized within the SCN, but physiological roles have not been determined48–50. Galanin, an inhibitory neuropeptide with critical roles in regulation of sleep and feeding51, has demonstrated staining that may reside in projections to the SCN from other regions of the hypothalamus4.
Although a number of known, physiologically relevant neuropeptides were released in response to optic nerve stimulation, two anticipated neuropeptides, VIP and GRP, were not detected. VIP and GRP are established intrinsic SCN peptides with roles processing photic signals from the eye to the SCN clock30, 52. Several considerations may have contributed to this result. First, collection of these peptides from releasate may have been hindered a number of ways: 1) unusually strong VIP- and GRP- binding affinities to their respective receptors, VPAC2 and BB2 receptors, 2) tight synaptic junctions impeding release of VIP and/or GRP into the extracellular environment, and/or 3) rapid degradation post-release. Second, concentrations of VIP and/or GRP released in response to the brief, acute light signal-associated stimulus may be too low to capture and aggregate using SPE pipette sampling. Third, VIP and/or GRP release may occur, however, it may be downstream from the acute phase of the light response. VIP’s role in SCN synchronization and entrainment is via regulation of SCN electrical activity53, inhibitory synaptic transmission54, intracellular signal transduction, and clock gene expression31. This is supported by the observation that sustained Periodl expression (after 30 min), but not initial Periodl gene induction, is reduced in VIP-mutant mice31. A required role for VIP has been proposed in timing-specific, light-induced gene expression55. Recent studies suggest that GRP and VIP may play redundant and intermediate roles in light-induced phase shifting30. Thus, neither may contribute in the initial, more acute physiological response to a phase-shifting light stimulus. This might explain why neither of these two SCN peptides were detected in samples collected during the first 20 min following ON stimulation in the horizontal SCN slice preparation.
Glutamate and PACAP are co-stored in RHT terminals of the ipRGCs that innervate the SCN46. These neurochemicals are known to orchestrate the effects of environmental light signals from the retina via the RHT that induce time-of-day- specific phase shifting of the SCN circadian clock12–14. To assess whether the observed stimulus-induced peptide release is downstream from these known RHT neurotransmitters, we incubated the horizontal SCN slice with competitive antagonists of the NMDA glutamate receptor ((2R)-amino- 5-phosphonovaleric acid, APV) or the PACAP PAC1R receptor (PACAP 6–38) prior to electrical stimulation of the ON coupled with simultaneous collection of releasate for pep- tidomic analysis. When APV was applied during subjective daytime at CT 6, a significant suite of peptides was released in response to ON stimulation (n = 10; Supplementary Fig. 1A vs. B). However, the peptide composition of releasate was diminished by PACAP 6–38 administration at the same circadian time-point (n=4; Supplementary Fig. 1C). At CT 14, glutamate signaling via NMDA receptor activation has been implicated in delay of SCN clock phase12. No peptide release was detected at CT 14 when APV is administered during the electrical stimulus that alone effectively causes phase-shifting (n=4; Supplementary Fig. 1D). APV administration at CT 14 effectively blocks glutamate-induced phase delays12. Peptide release also was blocked when the selective inhibitor, PACAP 6–38 was present during both stimulus parameter conditions at CT 19 (n=4; Supplementary Fig. 1E and F). Blocking of PACAP signaling during direct glutamate stimulation has been shown to augment the glutamate-stimulated phase-advance in SCN brain slices56.
Although previous studies have reported differential peptide expression23–24, 57 and single peptide release58 from the hypothalamus under physiological conditions, our work is the first to demonstrate temporally specific suites of peptide release evoked by a single environmentally relevant stimulus. Previously reported SCN peptides include molecules that are both endogenously expressed within the SCN and those delivered to the SCN via afferent innervation. These peptides may act as modulators of SCN responses, relays during intra-SCN stimulus processing, and/or feed-forward output signals from the SCN that influence timing of downstream physiological processes. Further characterization of these observed peptides is necessary to understand their mechanistic implications in circadian regulation.
CONCLUSION
Our results are significant in two ways, one being methodological and the second providing new insights on the complex interplay of peptides governing circadian rhythms. From the methodological point of view, MS analysis of peptide releasate represents a direct and untargeted discovery strategy that can be adapted to a number of brain systems. While our results demonstrate surprisingly complex peptide release profiles, a number of variables prevent this approach from being comprehensive. High-affinity peptide/receptor binding, low-concentration release, rapid extracellular degradation, and biophysical properties may result in peptide quantities below detection limits. In addition, the amount of peptide detected precludes most peptide identification approaches, so that our current study relied on matching our release profiles to more comprehensive LC-MS-based peptidomics measurements.
From the neuroscience perspective, the results of our study highlight qualitative differences in secreted neuropeptide profiles across circadian time-points in response to stimuli that induce physiological state-changes. The peptide release profiles are surprisingly rich. The results suggest that SCN physiology is modulated by differential peptide release of both known and unexpected peptides that communicate time-of-day-specific photic signals via previously unreported neuropeptide signatures. Our findings validate the horizontal SCN slice preparation as a model system and inform future research toward exploring previously uncharacterized SCN neuropeptides and their prospective contributions to mammalian circadian-timing homeostasis.
METHODS
Animals
This study was conducted using male Long-Evans rats (BluGill), seven- to twelve-weeks old. This inbred strain has been analyzed via dense genomic scan (10 cM inter-marker interval), confirming only one allele at each locus examined59. Male animals were entrained to a 12h:12h light/dark cycle and provided food and water ad libitum. Circadian time (CT) in SCN brain slices reflects the light schedule that the animals were entrained to prior to sacrifice, in which the time of “lights on” is designated as CT 0. All animal care and procedures were approved by the University of Illinois at Urbana-Champaign Laboratory Animal Care Advisory Committee, in full compliance with federal animal care guidelines.
SCN Brain Slice Preparation
Animals were disoriented and sacrificed by decapitation during subjective daytime (CT 0 – 10). Horizontal brain slices (500 μm) with attached ONs (Fig. 1) were prepared using a combination of tissue chopper and Vibratome (Leica Microsystems). Tissue slices were maintained in a brain slice chamber perfused with phenol red-free Earle’s Balanced Salt Solution (EBSS), supplemented with 24.6 mM glucose, 26.2 mM NaHCO3, and 2.5 mg/l gentamicin, and saturated with 95% 02/5% CO2 at 37°C, pH 7.4.
Optic nerve stimulation
Electrical stimulation of the ON was performed at circadian time (CT) 6, 14, or 19. These circadian times in the free-running SCN in vitro correspond to the entrained animals’ subjective daytime, early night, and late night, respectively. In animals, the circadian system is “blind’ to light experienced in daytime, even in ambient darkness, whereas at the later two CTs, which occur during nighttime, light stimulates phase-resetting41–42, 5, 60. Non-photic signals can alter SCN phasing in daytime41–42, 55, 60. Accordingly, effects of stimulation applied to both optic nerves (ONs) were examined at each CT using paired suction electrodes at a distance ~ 4 mm distal to the optic chiasm. Effective voltage, frequency, and pulse duration parameters were employed at respective stimulation times and delivered for 5 min using a Grass isolated-pulse stimulator (Grass Technologies). For tissue used in electrophysiological recording, ONs were gently released from the suction electrode immediately following stimulation. When releasate was collected, suction electrodes remained attached to the ONs until the end of releasate collection (15 min following termination of stimulation).
In some experiments, the horizontal SCN slice was incubated for 10 min with either a competitive antagonist of the NMDA glutamate receptor, (2R)-amino-5-phosphonovaleric acid (APV, 100 μM) or the competitive PAC1R antagonist, PACAP 6–38 (10 μM), before stimulating the ON and collecting releasate. Effects of these inhibitors of ON signaling on peptide release were evaluated at CT 6, 14, or 19.
Electrophysiological Recording
During the circadian cycle subsequent to stimulation, single-unit spontaneous activity of SCN neurons was recorded extracellularly 12. Activity of each neuron was averaged across a 4-min window. A 2-h binned running average of randomly sampled single-neuron firing activity was recorded using an in-house program developed in LabView (Laboratory Technologies). For each experiment, averages of firing rates and standard errors were plotted versus timeof-day to determine the CT of peak activity for the SCN neuronal population. Statistical analysis of electrophysiological results was performed using one-way ANOVA with Dunnett post-hoc test to compare experimental groups to control.
SPE pipette sampling of releasates
The approach employed for direct peptidomics of the SCN is outlined in Fig. 1, and has been described previously38. Briefly, C18 Zip Tip™ pipettes (Millipore Co., Billerica, MA) were “wetted” by aspiration with 50% ACN and equilibrated in EBSS immediately prior to use. The wetted pipettes were connected via Tygon tubing (Saint-Gobain Performance Plastics Co., Paris, FR) to a Harvard Apparatus (Harvard Biosience, Holliston, MA) syringe pump and Hamilton gas-tight syringe (Hamilton Company, Reno, NV). The ZipTip was mounted on a micromanipulator for precise positioning above the SCN on the surface of the brain slice. The inner diameter of the pipette tip measured 500 μm and was sufficient to cover the surface area of SCN tissue. Collection was performed by running the pump in negative mode, pulling extracellular samples across the C18 material at a rate of 0.2 μL/min. Pipette collections, both pre-stimulation and stimulation-onset, lasted 20 min unless otherwise noted.
Following sample collection, ZipTips removed, cleared of EBSS and aspirated with Millipore H2O to rinse salts. Peptide samples were eluted with 2 μl of 70% ACN onto MALDI target plates prespotted with cyano-4 hydroxycinnamic acid (HCCA) MALDI matrix (PAC384 anchor-chips™, Bruker). Eluate droplets were maintained within pre-de- fined sample spot regions on the target plate and allowed to dry at room temperature prior to analyses.
MALDI TOF MS
MALDI TOF MS (MALDI time-of-flight MS) was performed using an Ultraflex II (Bruker Daltonics) mass spectrometer which was equipped with pulsed nitrogen lasers for desorption/ionization and coupled to time-of-flight tubes and detectors with high-speed digitizer/analyzers. Mass spectra were obtained using positive, reflectron mode. Mass calibration was performed using the pre-spotted calibrant mixture included with the Bruker PAC anchor-chips. Mass accuracies typically were observed to be within 100 ppm. Spectra represent the accumulation of 300 shots. Following data collection, the mass spectra were smoothed and baseline corrected.
Because the amount of peptide collected and measured using this approach precludes identification via tandem MS, the information on the peptides involves our accurate mass values. However, we have performed a number of studies on the peptides within the SCN using tissue punches and tandem MS that have identified detailed and accurate lists of prohormone-derived peptides23–24, 38, 40. Here, our neuropeptide identifications were inferred from intact mass values observed in MS1 data via MALDI TOF MS analyses and matching them to our curated lists of confirmed SCN peptides with a mass accuracy within 100 ppm.61
Supplementary Material
ACKNOWLEDGMENTS
The project described was supported by Award No. P30 DA018310 from the National Institute on Drug Abuse (JVS), the National Heart, Lung and Blood Institute under Award No. HL092571 (MUG), and the National Science Foundation under Award No. IOS-1354913 (MUG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
REFERENCES
- 1.Hastings MH; Maywood ES; Reddy AB, Two decades of circadian time. J Neuroendocrinol 2008, 20 (6), 812–9. [DOI] [PubMed] [Google Scholar]
- 2.Robinson I; Reddy AB, Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett 2014, 588 (15), 2477–83. [DOI] [PubMed] [Google Scholar]
- 3.Rosenwasser AM; Turek FW, Neurobiology of Circadian Rhythm Regulation. Sleep Med Clin 2015, 10 (4), 403–12. [DOI] [PubMed] [Google Scholar]
- 4.Abrahamson EE; Moore RY, Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 2001, 916 (1–2), 172–91. [DOI] [PubMed] [Google Scholar]
- 5.Welsh DK; Takahashi JS; Kay SA, Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 2010, 72, 551–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin LP; Allen CN, The circadian visual system, 2005. Brain Res Rev 2006, 51 (1), 1–60. [DOI] [PubMed] [Google Scholar]
- 7.Golombek DA; Rosenstein RE, Physiology of circadian entrainment Physiol Rev 2010, 90 (3), 1063–102. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez DC; Chang YT; Hattar S; Chen SK, Architecture of retinal projections to the central circadian pacemaker. Proc Natl Acad Sci U S A 2016, 113 (21), 6047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do MT; Yau KW, Adaptation to steady light by intrinsically photosensitive retinal ganglion cells. Proc Natl Acad Sci US A 2013, 110 (18), 7470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannibal J; Georg B; Hindersson P; Fahrenkrug J, Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci 2005, 27 (2), 147–55. [DOI] [PubMed] [Google Scholar]
- 11.Wong KY; Dunn FA; Berson DM, Photoreceptor adaptation in intrinsically photosensitive retinal ganglion cells. Neuron 2005, 48 (6), 1001–10. [DOI] [PubMed] [Google Scholar]
- 12.Ding JM; Chen D; Weber ET; Faiman LE; Rea MA; Gillette MU, Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science 1994, 266 (5191), 1713–7. [DOI] [PubMed] [Google Scholar]
- 13.Hannibal J, Roles of PACAP-containing retinal ganglion cells in circadian timing. Int Rev Cytol 2006, 251, 1–39. [DOI] [PubMed] [Google Scholar]
- 14.Beaule C; Mitchell JW; Lindberg PT; Damadzic R; Eiden LE; Gillette MU, Temporally restricted role of retinal PACAP: integration of the phase-advancing light signal to the SCN. J Biol Rhythms 2009, 24 (2), 126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell JW; Atkins N Jr.; Sweedler JV; Gillette MU, Direct cellular peptidomics of hypothalamic neurons. Front Neuroendocrinol 2011, 32 (4), 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lokshin M; LeSauter J; Silver R, Selective Distribution of Retinal Input to Mouse SCN Revealed in Analysis of Sagittal Sections. J Biol Rhythms 2015, 30 (3), 251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long MA; Jutras MJ; Connors BW; Burwell RD, Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci 2005, 8 (1), 61–6. [DOI] [PubMed] [Google Scholar]
- 18.Wang MH; Chen N; Wang JH, The coupling features of electrical synapses modulate neuronal synchrony in hypothalamic superachiasmatic nucleus. Brain Res 2014, 1550, 9–17. [DOI] [PubMed] [Google Scholar]
- 19.Albers HE; Walton JC; Gamble KL; McNeill J. K. t.; Hummer DL, The dynamics of GABA signaling: Revelations from the circadian pacemaker in the suprachiasmatic nucleus. Front Neuroendocrinol 2017, 44, 35–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diemer T; Landgraf D; Noguchi T; Pan H; Moreno JL; Welsh DK, Cellular circadian oscillators in the suprachiasmatic nucleus remain coupled in the absence of connexin-36. N euroscience 2017, 357, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver R; LeSauter J; Tresco PA; Lehman MN, A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 1996, 382 (6594), 810–3. [DOI] [PubMed] [Google Scholar]
- 22.Moore RY; Speh JC; Leak RK, Suprachiasmatic nucleus organization. Cell Tissue Res 2002, 309 (1), 89–98. [DOI] [PubMed] [Google Scholar]
- 23.Lee JE; Zamdborg L; Southey BR; Atkins N Jr.; Mitchell JW; Li M; Gillette MU; Kelleher NL; Sweedler JV, Quantitative peptidomics for discovery of circadian-related peptides from the rat suprachiasmatic nucleus. J Proteom e Res 2013, 12 (2), 585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Southey BR; Lee JE; Zamdborg L; Atkins N Jr.; Mitchell JW; Li M; Gillette MU; Kelleher NL; Sweedler JV, Comparing label-free quantitative peptidomics approaches to characterize diurnal variation of peptides in the rat suprachiasmatic nucleus. Anal Chem 2014, 86 (1), 443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmar AJ, An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol 2003, 15 (4), 335–8. [DOI] [PubMed] [Google Scholar]
- 26.Atkins N Jr.; Mitchell JW; Romanova EV; Morgan DJ; Cominski TP; Ecker JL; Pintar JE; Sweedler JV; Gillette MU, Circadian integration of glutamatergic signals by little SAAS in novel suprachiasmatic circuits. PLoS One 2010, 5 (9), e12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannibal J; Georg B; Fahrenkrug J, PAC1- and VPAC2 receptors in light regulated behavior and physiology: Studies in single and double mutant mice. PLoS One 2017, 12 (11), e0188166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi Y, Arginine vasopressin signaling in the suprachiasmatic nucleus on the resilience of circadian clock to jet lag. Neurosci Res 2017. [DOI] [PubMed] [Google Scholar]
- 29.Ischia J; Patel O; Shulkes A; Baldwin GS, Gastrin-releasing peptide: different forms, different functions. Biofactors 2009, 35 (1), 69–75. [DOI] [PubMed] [Google Scholar]
- 30.Chan RK; Sterniczuk R; Enkhbold Y; Jeffers RT; Basu P; Duong B; Chow SL; Smith VM; Antle MC, Phase shifts to light are altered by antagonists to neuropeptide receptors. Neuroscience 2016, 327, 115–24. [DOI] [PubMed] [Google Scholar]
- 31.Vosko A; van Diepen HC; Kuljis D; Chiu AM; Heyer D; Terra H; Carpenter E; Michel S; Meijer JH; Colwell CS, Role of vasoactive intestinal peptide in the light input to the circadian system. Eur J Neurosci 2015, 42 (2), 1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamble KL; Allen GC; Zhou T; McMahon DG, Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci 2007, 27 (44), 12078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed HE; Meyer-Spasche A; Cutler DJ; Coen CW; Piggins HD, Vasoactive intestinal polypeptide (VIP) phase- shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci 2001, 13 (4), 839–43. [DOI] [PubMed] [Google Scholar]
- 34.Sterniczuk R; Yamakawa GR; Pomeroy T; Antle MC, Phase delays to light and gastrin-releasing peptide require the protein kinase A pathway. Neurosci Lett 2014, 559, 24–9. [DOI] [PubMed] [Google Scholar]
- 35.Gelman JS; Wardman J; Bhat VB; Gozzo FC; Fricker LD, Quantitative peptidomics to measure neuropeptide levels in animal models relevant to psychiatric disorders. Methods Mol Biol 2012. , 829, 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L; Sweedler JV, Peptides in the brain: mass spectrometry-based measurement approaches and challenges. Annu Rev Anal Chem (Palo Alto Calif) 2008, 1, 451–83. [DOI] [PubMed] [Google Scholar]
- 37.Romanova EV; Sweedler JV, Peptidomics for the discovery and characterization of neuropeptides and hormones. Trends Pharm acol Sci 2015, 36 (9), 579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatcher NG; Atkins N Jr.; Annangudi SP; Forbes AJ; Kelleher NL; Gillette MU; Sweedler JV, Mass spectrometry-based discovery of circadian peptides. Proc Natl Acad Sci U S A 2008, 105 (34), 12527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bora A; Annangudi SP; Millet LJ; Rubakhin SS; Forbes AJ; Kelleher NL; Gillette MU; Sweedler JV, Neuropeptidomics of the supraoptic rat nucleus. J Proteom e Res 2008, 7 (11), 4992–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JE; Atkins N Jr.; Hatcher NG; Zamdborg L; Gillette MU; Sweedler JV; Kelleher NL, Endogenous peptide discovery of the rat circadian clock: a focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol Cell Proteom ics 2010, 9 (2), 285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannibal J; Ding JM; Chen D; Fahrenkrug J; Larsen PJ; Gillette MU; Mikkelsen JD, Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 1997, 17 (7), 2637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hannibal J; Ding JM; Chen D; Fahrenkrug J; Larsen PJ; Gillette MU; Mikkelsen JD, Pituitary adenylate cyclase activating peptide (PACAP) in the retinohypothalamic tract: a daytime regulator of the biological clock. Ann N YAcad Sci 1998, 865, 197–206. [DOI] [PubMed] [Google Scholar]
- 43.Burgoon PW; Gillette MU In Optic nerve stimulation can cause phase advances in suprachiasm atic nucleus activity during the daytime and during the late night, Society for Neuroscience Annual Meeting, New Orleans, LO, New Orleans, LO, 2000. [Google Scholar]
- 44.Dardente H; Menet JS; Challet E; Tournier BB; Pevet P; Masson-Pevet M, Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res 2004, 124 (2), 143–51. [DOI] [PubMed] [Google Scholar]
- 45.Gillette MU; Reppert SM, The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull 1987, 19 (1), 135–9. [DOI] [PubMed] [Google Scholar]
- 46.Hannibal J; Moller M; Ottersen OP; Fahrenkrug J, PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol 2000, 418 (2), 147–55. [PubMed] [Google Scholar]
- 47.Brown TM; McLachlan E; Piggins HD, Angiotensin II regulates the activity of mouse suprachiasmatic nuclei neurons. Neuroscience 2008, 154 (2), 839–47. [DOI] [PubMed] [Google Scholar]
- 48.Hong HK; Chong JL; Song W; Song EJ; Jyawook AA; Schook AC; Ko CH; Takahashi JS, Inducible and reversible Clock gene expression in brain using the tTA system for the study of circadian behavior. PLoS Genet 2007, 3 (2), e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahata SK; Mahata M; Marksteiner J; Sperk G; Fischer-Colbrie R; Winkler H, Distribution of mRNAs for Chromogranins A and B and Secretogranin II in Rat Brain. Eur J Neurosci 1991, 3 (9), 895–904. [DOI] [PubMed] [Google Scholar]
- 50.Oh SW; Harris JA; Ng L; Winslow B; Cain N; Mihalas S; Wang Q; Lau C; Kuan L; Henry AM; Mortrud MT; Ouellette B; Nguyen TN; Sorensen SA; Slaughterbeck CR; Wakeman W; Li Y; Feng D; Ho A; Nicholas E; Hirokawa KE; Bohn P; Joines KM; Peng H; Hawrylycz MJ; Phillips JW; Hohmann JG; Wohnoutka P; Gerfen CR; Koch C; Bernard A; Dang C; Jones AR; Zeng H, A mesoscale connectome of the mouse brain. Nature 2014, 508 (7495), 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan LM; Spindel ER; Isselbacher KJ; Chin WW, Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A 1988, 85 (4), 1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shinohara K; Tominaga K; Isobe Y; Inouye ST, Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci 1993, 13 (2), 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermanstyne TO; Simms CL; Carrasquillo Y; Herzog ED; Nerbonne JM, Distinct Firing Properties of Vasoactive Intestinal Peptide-Expressing Neurons in the Suprachiasmatic Nucleus. J Biol Rhythms 2016. , 31 (1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itri J; Colwell CS, Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol 2003, 90 (3), 1589–97. [DOI] [PubMed] [Google Scholar]
- 55.Dragich JM; Loh DH; Wang LM; Vosko AM; Kudo T; Nakamura TJ; Odom IH; Tateyama S; Hagopian A; Waschek JA; Colwell CS, The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci 2010, 31 (5), 864–75. [DOI] [PubMed] [Google Scholar]
- 56.Chen D; Buchanan GF; Ding JM; Hannibal J; Gillette MU, Pituitary adenylyl cyclase-activating peptide: a pivotal modulator of glutamatergic regulation of the suprachiasmatic circadian clock. Proc Natl Acad Sci US A 1999, 96 (23), 13468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gouraud SS; Heesom K; Yao ST; Qiu J; Paton JF; Murphy D, Dehydration-induced proteome changes in the rat hypothalamo-neurohypophyseal system. Endocrinology 2007, 148 (7), 3041–52. [DOI] [PubMed] [Google Scholar]
- 58.Watanobe H, In vivo release of prolactin-releasing peptide in rat hypothalamus in association with luteinizing hormone and prolactin surges. Neuroendocrinology 2001, 74 (6), 359–66. [DOI] [PubMed] [Google Scholar]
- 59.Tischkau SA; Mitchell JW; Pace LA; Barnes JW; Barnes JA; Gillette MU, Protein kinase G type II is required for night-to-day progression of the mammalian circadian clock. Neuron 2004, 43 (4), 539–49. [DOI] [PubMed] [Google Scholar]
- 60.Webb IC; Coolen LM; Lehman MN, NMDA and PACAP receptor signaling interact to mediate retinal-induced scn cellular rhythmicity in the absence of light. PLoS One 2013, 8 (10), e76365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falth M; Skold K; Norrman M; Svensson M; Fenyo D; Andren PE, SwePep, a database designed for endogenous peptides and mass spectrometry. Mol Cell Proteom ics 2006, 5 (6), 998–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


