Figure 1. Identification of EPN-selective FDA-approved oncology drug candidates using in vitro and in silico screening.
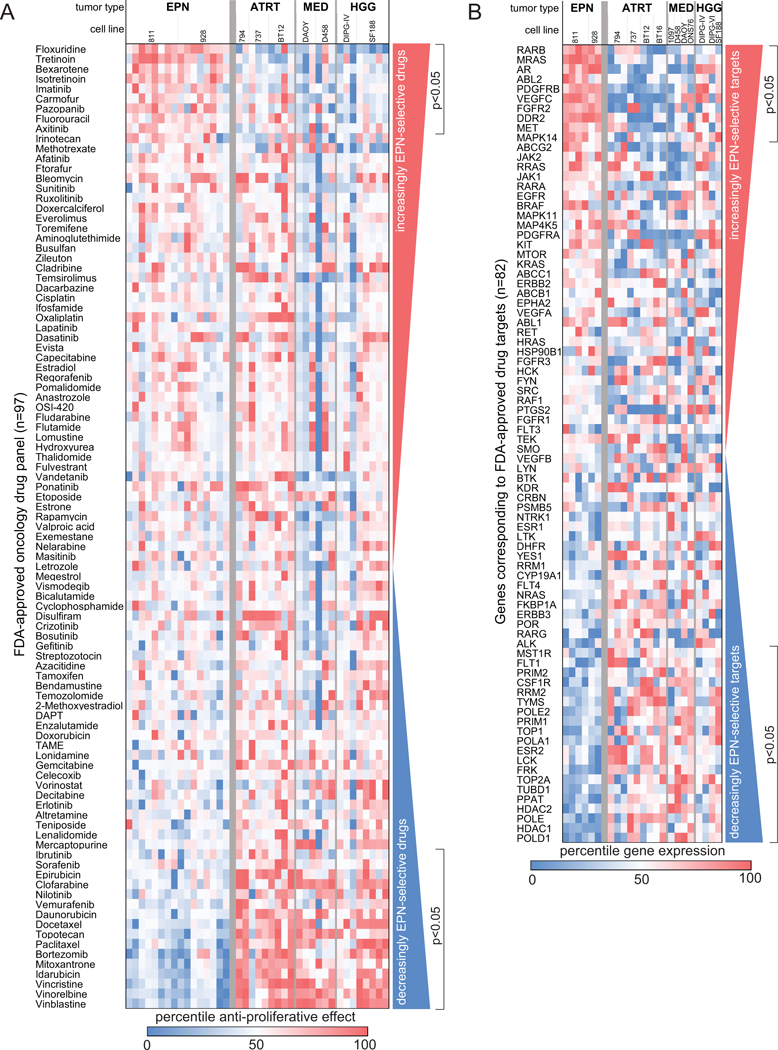
(A) Comparative drug sensitivity analysis of 97 FDA approved oncology drugs ranked from most EPN-selective (top) to most EPN non-selective (bottom). Treatment effect was measured using proliferation assays (tritiated thymidine incorporation) in a panel of pediatric brain tumor cell lines listed as follows with number of replicates in parenthesis (EPNs 811 (8) and 928 (8); ATRTs 794(2), 737 (3) and BT12 (4); MEDs DAOY (3) and D458 (3); HGGs DIPG-IV (3) and SF199 (5)). (B) In silico drug screening for putative targets of FDA-approved oncology drugs. RNA expression of 82 target genes was compared between EPN cell lines and other pediatric brain tumor cells lines to identify EPN-selective (top) and non-selective (bottom) drug targets. Cell lines included in this analysis, with replicates in parenthesis, were EPNs 811 (4) and 928 (2), ATRTs 794 (3), 737, BT12 and BT16 (2 each), MEDs 1097, D458, DAOY and ONS76 (1 each) and HGGs DIPG-IV (2), DIPG-VI and SF188 (1 each).
