Figure 3. The blockage of dNTPase activity of SAMHD1 retains co-expression of Foxp3 and Helios in human Tregs.
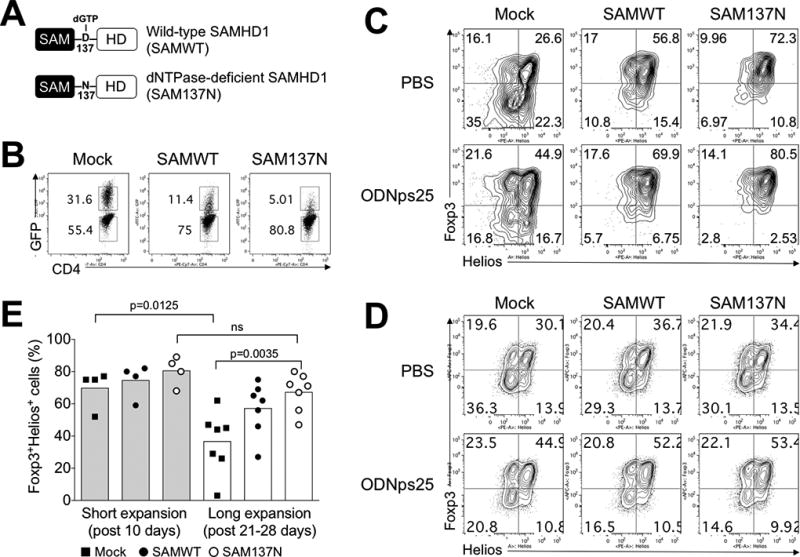
(A) Expression constructs of Wild-type SAMHD1 (SAMWT) and dNTPase-deficient SAMHD1 (SAM137N). To construct retroviral SAMWT and SAM137N, HA-SAMHD1 and HA-SAMHD1 D137N (kindly gifted from Dr. Welbourn, NIAID, NIH) was re-cloned in retroviral expression vector (pRetro-X-IRES-Zsgreen1), and produced viral particles for amphotropic transduction in Tregs. (B) Transduction efficiency of in SAMWT and SAM137N. The preparation and expansion protocol were described in Methods. Briefly, FACS-sorted Tregs from healthy donors (n=4 for short expansion; n=7 for long expansion) were stimulated with anti-CD3ε/CD28 antibodies, and transduced with retroviral Mock vector (Mock), SAMWT, and SAM137N. GFP expression was measured at day 19 after transduction. The number in the plot indicates the frequency of GFP+ and GFP− Tregs, respectively. (C) Stabilization of Foxp3 and Helios expression in GFP+ SAMWT and SAM137N Tregs. Foxp3 and Helios were measured by intracellular FACS staining after short- and long-term expansion periods, respectively. Data are presented as the mean of MFI in each donors. The p values were calculated by two-tailed Student’s t-test. (D) The expression of Foxp3 and Helios in GFP− Tregs. (E) ODNps25 effect to stabilize Foxp3 and Helios expressions in SAMHD1 construct-overexpressed Tregs. Transduction and expansion culture of Tregs was same as described in Fig. 3C in the presence of ODNps25 (2 µM) or not. FACS analysis is performed by gating of intercellular stained GFP+ transduced cells. Percent of Foxp3 and Helios expression is indicated in quadrant gate. Data in C and D is one of seven independent experiments with different donors.
