Abstract
The liquid biopsy of ascites fluid could be an excellent source of tumor and microenvironment for the study of prognostic biomarkers because of its accessibility. Tumor‐infiltrating lymphocytes (TILs) can predict prognosis in multiple malignancies, including the response to immune checkpoint inhibitors, a breakthrough cancer therapy. However, TILs’ profiles from malignant ascites have not been extensively studied. Using flow cytometric analysis, we quantified the proportion of exhausted T cells and memory/naive/effector T‐cell subsets, among the CD4+ and CD8+ T‐cell populations of paired TILs and peripheral blood T cell samples (n = 22). The correlation between CD4+ and CD8+ subset profiles suggested that the combined analysis of CD4+ and CD8+ cells in malignant ascites was clinically significant. We found that cells positive for the exhaustion markers programmed cell death‐1 (PD‐1), and T‐cell immunoglobulin and mucin domain 3 (TIM‐3), and cells coexpressing PD‐1 and TIM‐3 abundantly exist among malignant ascites TILs. Furthermore, patients with high frequency of PD‐1+ TIM‐3+ cells among the CD4+ and CD8+ T‐cell population showed worse clinical outcome in multivariate analysis (n = 27). We propose that exhausted ascites TILs represent a clinically significant prognostic biomarker in advanced gastrointestinal cancer and represent an important target for immune checkpoint inhibitors.
Keywords: ascites, exhaustion, immune checkpoint, PD‐1, tumor‐infiltrating lymphocytes
1. INTRODUCTION
In advanced gastrointestinal cancer, patients often suffer from malignant ascites as a result of peritoneal dissemination of cancer cells.1 In various malignancies, ascites is a sign of advanced disease and poor prognosis with only 11% of patients surviving longer than 6 months.2 Malignant ascites consists of cancer cells, mesothelial cells, fibroblasts, and inflammatory cells including abundant T cells, natural killer (NK) cells, B cells, macrophages, and neutrophils. Each of these cells produces various cytokines that regulate the phenotype and function of components, leading to formation of tumor microenvironment in malignant ascites.1
However, the characteristics of tumor‐infiltrating lymphocytes (TILs) within ascites, which should be the potential sampling resource for translational research because of its easy access, have not been fully understood. Accumulating evidence has shown the clinical significance of TILs of the primary tissues as a therapeutic target and biomarker. In this study, we evaluated the character of ascites TILs, to elucidate the cells that constitute ascites TILs and to clarify their function. Ascites represent an alternative resource for TILs of the primary tissues and distinct from lymphocytes in peripheral blood (PB).
2. MATERIALS AND METHODS
Patients, samples, and ethical considerations
All patients diagnosed with malignant ascites of gastrointestinal cancer, regardless of previous treatment history, were eligible for inclusion in this observational study. Ascites and peripheral blood (PB) T cells were collected from Apr 2015 to Apr 2017, and a total of 27 patients were recruited. All studies were performed in accordance with the Declaration of Helsinki principals and the guidelines of the Research Ethics Committee on Human Experimentation of Kyushu University (#28‐95), and written informed consent was received from all patients.
Isolation of mononuclear cells from peripheral blood and ascites TILs
PB mononuclear cells were obtained by standard gradient centrifugation using Ficoll‐Paque PLUS (GE Healthcare). Ascites TILs obtained from patients were immediately centrifuged for 5 minutes at 1500 rpm. Cell pellets were then washed and re‐suspended in 20 mL of PBS. The cell suspension was then layered on 5 mL of Ficoll‐Paque PLUS, before gradient centrifugation as performed for PB.
Statistical analysis
Statistical analysis was carried out with JMP software (version 11.0, SAS institute). Student's t test was performed to compare two groups. Correlation analysis was calculated using the Spearman's statistic. Cox proportional hazards model for univariate and multivariate analysis was performed to calculate adjusted hazard ratios (HR) and their 95% confidence intervals (CI). The cut‐off value was determined by the median of the variables. Variables with a P value of less than 0.05 in univariate analysis were tested in the multivariate analysis. The Kaplan‐Meier method, with log‐rank test, was used to evaluate overall survival. Statistically significant differences are indicated by asterisks (*P < .05, **P < .01, ***P < .001).
More detailed version of methods are included in Data S1.
3. RESULTS
To investigate the characteristics of ascites TILs, we undertook multicolor FACS of paired ascites TILs and PB T cells from 22 patients (Table S1) that were identical based on consensus immune phenotyping.3 Although obvious differences in the frequency of CD3+ T cells (PB, 31.5%; ascites TILs, 36.4%), CD19+ B cells (PB, 3.6%; ascites TILs, 3.7%), and CD56+ NK cells (PB, 8.3%; ascites TILs, 7.8%) between ascites TILs and PB T cells were not observed (Figure S1), there were distinct differences in the frequency of each T‐cell subset. A higher frequency of CD45RA− memory T cells among CD4+ T cells was found for ascites TILs than PB T cells (PB, 42.9%; ascites TILs, 72.0%). Similar results were seen for CD8+ T cells (PB, 26.2%; ascites TILs, 40.2%) (Figure S2). The frequency of naïve T cells showed a reverse pattern to CD45RA− memory T cells, in that the frequency of C‐C chemokine receptor (CCR)7 CD45RA+ T cells was lower for CD4+ (PB, 47.9%; ascites TILs, 18.7%) and CD8+ ascites TILs (PB, 26.4%; ascites TILs, 10.7%) (Figure S2). This correlation between CD4+ and CD8+ ascites TIL profiles, and the high memory/low naïve T‐cell frequencies among ascites TILs, suggests that ascites TILs have been exposed to antigen and have survived for long periods.
In addition, there were significant differences in the other CD4+ helper T‐cell subsets including CD4+ CCR6 C‐X‐C chemokine receptor (CXCR)3+ type1 helper T cells (Th1) (PB, 17.0%; ascites TILs, 34.4%), CD4+ CCR6− CXCR3− type2 helper T cells (Th2) cells (PB, 56.9%; ascites TILs, 29.9%), CD4+ CD45RA− CXCR5+ follicular helper T cells (Tfh) (PB, 11.4%; ascites TILs, 7.9%), and CD4+ CD25+ CD127− regulatory T cells (Treg) (PB, 3.4%; ascites TILs, 6.3%) between PB and ascites TILs (Figure S1c,d). The clear differences in each T‐cell subset, memory, naive, effector, Th1/2, Tfh, and Treg, show that ascites TILs possess distinct characteristics compared to PB T cells.
Recent advances in cancer research have led to the development of immune checkpoint inhibitors for use in multiple malignancies.4 These agents revitalize antitumor T‐cell activity through the inhibition of immune checkpoints, such as programmed cell death‐1 (PD‐1) and T‐cell immunoglobulin and mucin domain 3 (TIM‐3), which are considered as exhaustion markers for T cells. Exhausted T cells, highly expressing inhibitory receptors, produce fewer effector cytokines and lose the ability to eliminate cancer.5 To investigate the exhaustion status of ascites TILs, we explored the exhaustion markers of ascites TILs and paired PB T cells (Table S1, n = 22). Interestingly, the frequency of PD‐1+ cells was higher for CD4+ (PB, 26.1%; ascites TILs, 56.0%) and CD8+ (PB, 33.0%; ascites TILs, 45.9%) ascites TILs (Figure 1A,B). Similarly, TIM‐3+ cells were also enriched among CD4+ (PB, 2.8%; ascites TILs, 8.7%) and CD8+ (PB, 6.1%; ascites TILs, 11.1%) ascites TILs (Figure 1A,B). The expression of inhibitory molecules is reported to reflect the degree of T‐cell exhaustion,6 and coexpression of PD‐1 and TIM‐3 indicated the exhausted status of T cells in terms of proliferation and cytokine production.7, 8, 9 In our study, PD‐1+ TIM‐3+ cells were predominantly observed in CD4+ (PB, 1.6%; ascites TILs, 5.8%) and CD8+ (PB, 3.4%; ascites TILs, 6.0%) ascites TILs, compared with PB T cells (Figure 1A,B). These results indicate that ascites TILs significantly express exhaustion markers that could be a target for anti‐PD‐1 or anti‐TIM‐3 antibody therapy in gastrointestinal malignant ascites.
Figure 1.
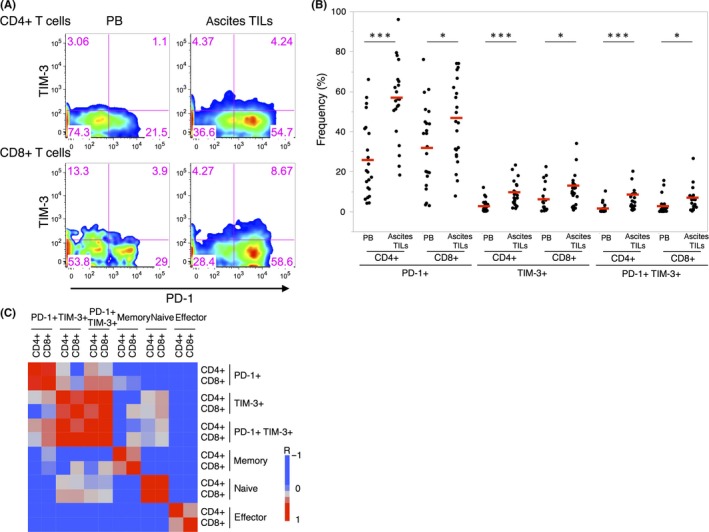
Exhaustion marker‐positive T cells are enriched among CD4+ and CD8+ ascites tumor‐infiltrating lymphocytes (TILs). A, Representative FACS plots of ascites TILs and paired peripheral blood (PB) T cells showing programmed cell death‐1 (PD‐1)+ and T‐cell immunoglobulin and mucin domain 3 (TIM‐3)+ cells within the CD4+ and CD8+ cells (case 62). B, Summarized dot plots of the frequency of exhaustion marker‐positive cells among ascites TILs and paired PB T cells (n = 22). Median values are indicated with red lines. *P < .05; ***P < .001. C, Correlation analysis of each subset, revealing similar signatures between CD4+ and CD8+ ascites TILs (n = 22)
To study the relationship between expression of the inhibitory molecules and memory status within ascites TILs, we quantified the frequencies of memory/naive/effector T cells within each fraction (PD‐1− TIM‐3−/PD‐1− TIM‐3+ /PD‐1+ TIM‐3−/PD‐1+ TIM‐3+) in CD4+ and CD8+ ascites TILs (n = 13) (Figure S3). Interestingly, PD‐1+ TIM‐3− and PD‐1+ TIM‐3+ fractions revealed higher frequencies of memory cells (CD4+ T cells: PD‐1− TIM‐3−, 74.5%; PD‐1− TIM‐3+, 56.1%; PD‐1+ TIM‐3−, 97.7%; PD‐1+ TIM‐3+, 94.3%; CD8+ T cells: PD‐1− TIM‐3−, 47.1%; PD‐1− TIM‐3+, 26.0%; PD‐1+ TIM‐3−, 82%; PD‐1+ TIM‐3+, 70.0%) and lower frequency of naïve cells (CD4+ T cells: PD‐1− TIM‐3−, 22.5%; PD‐1− TIM‐3+, 41.7%; PD‐1+ TIM‐3−, 0.6%; PD‐1+ TIM‐3+, 1.8%; CD8+ T cells: PD‐1− TIM‐3−, 8.6%; PD‐1− TIM‐3+, 25.7%; PD‐1+ TIM‐3−, 2.8%; PD‐1+ TIM‐3+, 4.4%). These results suggest that PD‐1+ cells in ascites TILs preferentially showing the memory phenotype might be caused by long‐term antigen exposure. This tendency between exhaustion marker expression and memory phenotype was almost equally observed in CD4+ and CD8+ T cells.
Next, we undertook a correlation analysis for each cell fraction to determine the relationship between CD4+ and CD8+ cells and found a significant correlation between CD4+ and CD8+ T cells in relation to exhaustion marker‐positive T cells (PD‐1: r = 0.6866, P = .0004; TIM‐3: r = 0.6147, P = .0023; PD‐1+ TIM‐3+: r = 0.7892, P < .0001) and memory/naive/effector T cells (memory: r = 0.5490, P = .0081; naive: r = 0.7146, P = .0002; effector: r = 0.5093, P = .0155; Figure 1C). Strong correlation between CD4+ and CD8+ T cells in each subset suggests that analysis of ascites TILs should be focused on both CD4+ and CD8+ T cells, although most TIL studies to date have focused intensively on CD8+ T cells. Additionally, patients with a high frequency of PD‐1+ cells in ascites TILs showed a high frequency of TIM‐3+ cells. These patients’ ascites TILs are likely to be exhausted, because coexpression of PD‐1 and TIM‐3 reflects dysfunction in both cytokine production and cancer elimination;6 it might, therefore, be important to stratify gastrointestinal malignant ascites patients on this basis.
Next, we explored the clinical significance of these exhaustion marker‐positive ascites TILs. As exhausted T cells are assumed to be in a dysfunctional state as the consequence of long‐term antigen exposure (Figure S3),6 we hypothesized that the frequency of exhaustion marker‐positive cells might be dependent on the time course of the disease. First, we evaluated the relationship between the frequency of PD‐1+ and TIM‐3+ cells and disease progression in gastrointestinal malignant ascites patients at two different time points (n = 4; Figure 2A). As expected, the frequency of PD‐1+, TIM‐3+, and PD‐1+ TIM‐3+ cells increased after the first biopsy among both the CD4+ and CD8+ cell populations (Figure 2B,C). This observation raised the possibility that the frequency of PD‐1+/TIM‐3+ ascites TILs could be a biomarker of patient prognosis. We then undertook univariate and multivariate analyses of the frequencies of T‐cell subsets and clinical variables to identify the prognostic marker in malignant ascites using expanded cohort (Table 1, n = 27). PD‐1+ TIM‐3+ cells in both CD4+ and CD8+ T cells (P = 0.0098; hazard ratio [HR], 0.2941; 95% confidence interval [CI], 0.1169‐0.7383), memory cells in CD4+ T cells (P = 0.0433; HR, 2.2843; 95% CI, 1.0236‐5.1644), and memory cells in both CD4+ and CD8+ T cells (P = 0.0156; HR, 2.8032; 95% CI, 1.2099‐7.7683) were significantly related with survival in univariate analysis (Table 1). In the multivariate analysis, PD‐1+ TIM‐3+ cells in both CD4 and CD8 T cells (P = 0.0325; HR, 0.3255; 95% CI, 0.1111‐0.9105) were shown to be the only significant prognostic variable (Table 1). Kaplan‐Meier survival analysis revealed that patients with high frequency of PD‐1+ TIM‐3+ cells among both CD4+ and CD8+ T cells showed worse clinical outcome (Figure 3A, B). However, stratification of patients using only CD4+ PD‐1+ TIM‐3+ or CD8+ PD‐1+ TIM‐3+ was not sufficient predictive biomarker in our cohort (Figure 3C,D). At the same time, the survival analysis of only PD‐1+ cells or TIM‐3+ cells among CD4+ or CD8+ T cells did not reveal clinical significance (Figure S4, S5). These results suggest that patients with exhaustion of both CD4+ and CD8+ ascites TILs possess distinguishing characters within our cohort. Additionally, patients with a high CD4+ or CD8+ memory T‐cell phenotype showed better prognosis (Figure S6), supporting the validity of our findings, as a number of reports have shown patients harboring high memory T cells in ascites TILs have a relatively good prognosis in various malignancies.10 These results collectively suggest that PD‐1+ TIM‐3+ ascites TILs are an excellent prognostic biomarker, and indicate the clinical importance of direct biopsy of ascites TILs.
Figure 2.
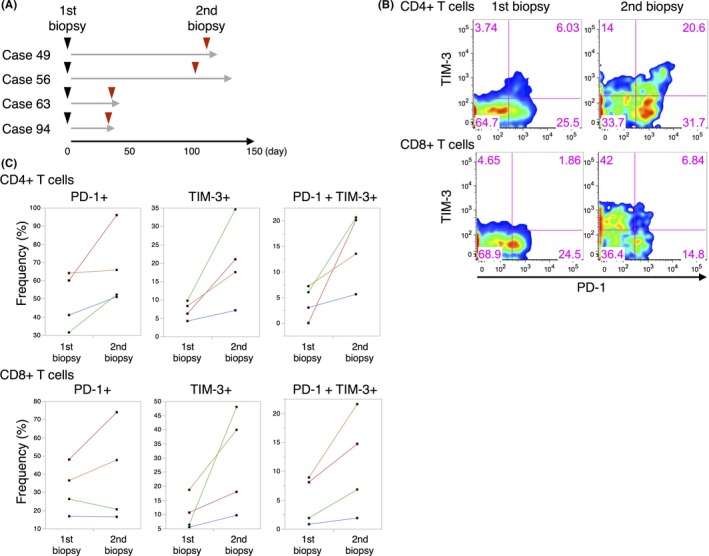
Exhaustion marker‐positive ascites tumor‐infiltrating lymphocytes (TILs) among CD4+ and CD8+ cells increases during disease progression. A, Schema of the experimental design. Arrows indicate time of biopsy and gray lines indicate the survival times of patients. B, Representative FACS plots of 1st and 2nd biopsy samples showing the proportion of programmed cell death‐1 (PD‐1)+ and T‐cell immunoglobulin and mucin domain 3 (TIM‐3)+ TILs among CD4+ and CD8+ T cells (case 56). C, Summarized dot plots of 1st and 2nd biopsy analyses in regard to the frequency of PD‐1+ and TIM‐3+ TILs among CD4+ and CD8+ T cells. Identical patients are indicated with colored lines (cases 49, 56, 63, and 94)
Table 1.
Univariate and multivariate analysis of survival among patients with gastrointestinal cancer, based on T‐cell subsets in malignant ascites
| Variable | HR (95% CI) | P‐value |
|---|---|---|
| Univariate analysis | ||
| CD4+ and CD8+ PD‐1+ TIM‐3+ | 0.2941 (0.1169‐0.7378) | 0.0098 |
| CD4+ and CD8+ memory | 2.8032 (1.2099‐7.7683) | 0.0156 |
| CD4+ PD‐1+ | 0.9362 (0.4306‐2.0666) | 0.8677 |
| CD4+ TIM‐3+ | 0.5270 (0.2321‐1.1839) | 0.1197 |
| CD4+ PD‐1+ TIM‐3+ | 0.4864 (0.2099‐1.0968) | 0.0822 |
| CD8+ PD‐1+ | 0.8582 (0.3932‐1.9023) | 0.7012 |
| CD8+ TIM‐3+ | 1.0735 (0.4907‐2.3475) | 0.8573 |
| CD8+ PD‐1+ TIM‐3+ | 0.8610 (0.3894‐1.8748) | 0.7052 |
| CD4+ memory | 2.2843 (1.0236‐5.1644) | 0.0433 |
| CD8+ memory | 1.8303 (0.8314‐4.0962) | 0.1324 |
| CD4+ naïve | 0.7966 (0.3628‐1.7497) | 0.5669 |
| CD8+ naïve | 1.7177 (0.7777‐3.7978) | 0.1783 |
| CD4+ effector | 0.5339 (0.2323‐1.2242) | 0.1364 |
| CD8+ effector | 0.5145 (0.2322‐1.1397) | 0.1004 |
| CD3+ | 0.9160 (0.3842‐2.1360) | 0.8387 |
| CD19+ B cells | 0.8832 (0.3708‐2.0583) | 0.7730 |
| CD56+ NK cells | 1.0755 (0.4684‐2.4943) | 0.8626 |
| Th1 (CCR6− CXCR3+) | 1.5627 (0.6046‐4.0402) | 0.3505 |
| Th2 (CCR6− CXCR3−) | 0.4967 (0.2081‐1.1728) | 0.1094 |
| Tfh (CXCR5+ CD45RA−) | 1.5627 (0.6046‐4.0402) | 0.3505 |
| Treg (CD25+ CD127−) | 0.9386 (0.3941‐2.1869) | 0.8830 |
| Age ≥63 years vs. <63 years | 0.7673 (0.3414‐1.6716) | 0.5060 |
| Sex, male vs. female | 0.4593 (0.1756‐1.0771) | 0.0745 |
| Histology differentiated adenocarcinoma vs. poorly differentiated adenocarcinoma | 0.5764 (0.2162‐1.4016) | 0.2286 |
| Cell number | 1.0398 (0.4419‐2.5023) | 0.9286 |
| Cancer type | ||
| Gastric vs. colorectal | 0.4114 (0.1410‐1.1842) | 0.0984 |
| Gastric vs. pancreatic | 0.7224 (0.2735‐1.9413) | 0.5099 |
| Colorectal vs. pancreatic | 1.7558 (0.6106‐5.1474) | 0.2923 |
| Multivariate analysis | ||
| CD4+ and CD8+ PD‐1+ TIM‐3+ | 0.3255 (0.1111‐0.9105) | 0.0325 |
| CD4+ and CD8+ memory | 3.0920 (0.7732‐10.9747) | 0.1046 |
| CD4+ memory | 0.6741 (0.1934‐2.705) | 0.5553 |
CCR, C‐C chemokine receptor; CI, confidence interval; CXCR, C‐X‐C chemokine receptor; HR, hazard ratio; NK, natural killer; PD‐1, programmed cell death‐1; Tfh, follicular helper T cell; Th1, type1 helper T cell; Th2, type2 helper T cell; TIM‐3, T‐cell immunoglobulin and mucin domain 3; Treg, regulatory T cell.
Figure 3.
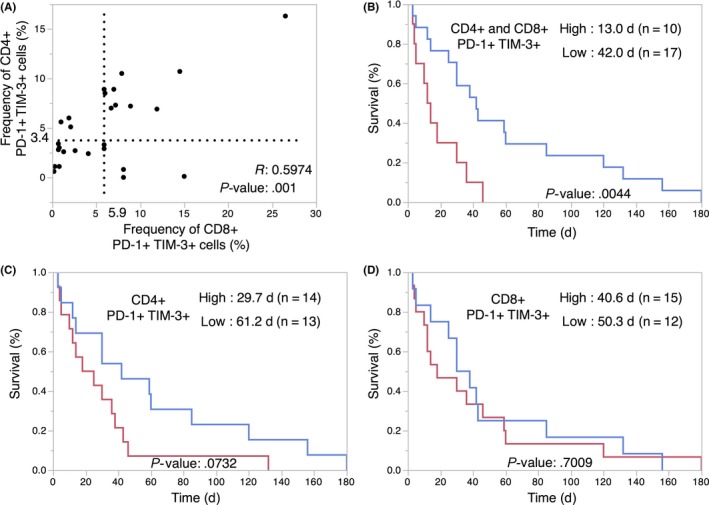
Programmed cell death‐1 (PD‐1)+ T‐cell immunoglobulin and mucin domain 3 (TIM‐3)+ exhausted ascites tumor‐infiltrating lymphocytes (TILs) within the CD4+ and CD8+ cell populations predict prognosis of gastrointestinal malignancies. A, Dot plot of PD‐1+ TIM‐3+ TILs among CD4+ and CD8+ T cells. The cut‐off value was defined by the median of the 27 cases (median CD4+, 3.4%; median CD8+, 5.9%). The correlation coefficient (r) and P value is indicated. B‐D, Kaplan‐Meier curves for overall survival of the indicated patient groups, as classified by the frequency of PD‐1+ TIM‐3+ cells among CD4+ and CD8+, CD4+, and CD8+ ascites TILs. Median overall survival (days) of each patient group is shown
4. DISCUSSION
In this study, we have shown the clinical significance of ascites TILs as a resource for translational medicine and the prediction of prognosis for gastrointestinal malignant ascites patients. Ascites TILs were characterized by a large proportion of PD‐1+ and TIM‐3+ exhausted T cells, strongly suggesting that immune checkpoint inhibitors should be indicated for patients with gastrointestinal malignant ascites who are intolerant of other cytotoxic drugs because of excessive ascites fluid. Furthermore, we clearly showed that these exhaustion marker‐positive cells showed mostly memory phenotype (Figure S3), which might suggest these cells reside for long periods in ascites. However, careful interpretation is required when we presume the functional phenotype of these PD‐1+ TIM‐3+ ascites TILs. Programmed cell death‐1 is both an activation marker and a key regulator of exhausted T cells. Although recent studies have reported a role for PD‐1 in preserving exhausted T cells from terminal differentiation,11 coexpression of PD‐1 and TIM‐3 has indicated the severe exhausted phenotype of T cells in proliferation and cytokine production.7, 8, 9 Further analysis is required to interpret the functional phenotype of these PD‐1+ TIM‐3+ ascites TILs. Our trial to reveal prognostic biomarkers in gastrointestinal malignant ascites patients based on T‐cell immune phenotyping in multivariate analysis proposed the significance of PD‐1+ TIM‐3+ ascites TILs. These findings are consistent with the finding that existence of PD‐1+ TIM‐3+ cells was associated with poor prognosis in renal cell carcinoma.12
T‐cell exhaustion has been intensively discussed in regard to CD8+ T cells, whereas the role of CD4+ exhausted T cells in the tumor microenvironment has not been fully evaluated.13 There are differences between exhausted CD4+ and CD8+ T cells regarding cytokine production and transcriptional networks;5 however, both play an important role in tumor elimination, and they interact with each other. Our data showing a correlation between CD4+ and CD8+ T cells in regard to the frequency of exhaustion marker and memory/naive/effector subsets, strongly suggests that there is a common phenotypic signature between CD4+ and CD8+ cells. Quantitative analysis of TILs by FACS enabled a detailed evaluation of each cell fraction and provided an opportunity for novel findings that might have been otherwise undetectable using conventional immunohistochemistry analysis. The observed relationship between exhausted T cells expressing PD‐1 and TIM‐3 among CD4+ helper and CD8+ cytotoxic T cells suggests that CD4+ helper T‐cell exhaustion is biologically significant.
Taken together, through immune phenotyping analyses of ascites TILs, we have shown that a large proportion of CD4+ and CD8+ T cells show an exhausted phenotype within gastrointestinal malignant ascites, and that this may therefore be both a therapeutic target and prognostic biomarker for the disease.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported in part by Japan Society for the Promotion of Science, and by Kyushu Medical Oncology Group (KMOG), a non‐profit organization.
Nakano M, Ito M, Tanaka R, et al. PD‐1+ TIM‐3+ T cells in malignant ascites predict prognosis of gastrointestinal cancer. Cancer Sci. 2018;109:2986–2992. 10.1111/cas.13723
Funding information
Japan Society for the Promotion of Science; Grant‐in‐Aid for Scientific Research (C) (to EB, #15K08970).
REFERENCES
- 1. Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330:337‐342. [DOI] [PubMed] [Google Scholar]
- 2. Parsons SL, Lang MW, Steele RJ, et al. Malignant ascites: a 2‐year review from a teaching hospital. Eur J Surg Oncol. 1996;22:237‐239. [DOI] [PubMed] [Google Scholar]
- 3. Maecker HT, McCoy JP, Nussenblatt R, et al. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol. 2012;12:191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zarour HM. Reversing T‐cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22:1856‐1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492‐499. [DOI] [PubMed] [Google Scholar]
- 7. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim‐3 and PD‐1 pathways to reverse T cell exhaustion and restore anti‐tumor immunity. J Exp Med. 2010;207:2187‐2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim‐3 and PD‐1 identifies a CD8+ T‐cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501‐4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim‐3 and PD‐1 in CD8 T‐cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733‐14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jia Q, Yang Y, Wan Y, et al. Tumor‐infiltrating memory T‐lymphocytes for prognostic prediction in cancer patients: a meta‐analysis. Int J Clin Exp Med. 2015;8:1803‐1813. [PMC free article] [PubMed] [Google Scholar]
- 11. Odorizzi PM, Pauken KE, Paley MA, Sharpe A, Wherry EJ. Genetic absence of PD‐1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212:1125‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granier C, Dariane C, Combe P, et al. Tim‐3 Expression on Tumor‐Infiltrating PD‐1(+)CD8(+) T Cells Correlates with Poor Clinical Outcome in Renal Cell Carcinoma. Cancer Res. 2017;77:1075‐1082. [DOI] [PubMed] [Google Scholar]
- 13. Jiang Y, Li Y, Zhu B. T‐cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


