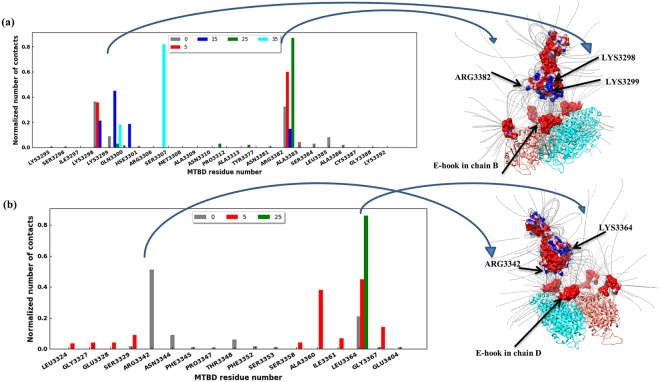
Figure 5.
Certain MTBD residues made most of the contacts with E-hooks. (a) Histogram of the number of E-hook B contacts each residue of the MTBD makes constants at various MTBD-microtubule distances (see legend). (b) Histogram of the number of E-hook D contacts each residue of the MTBD makes constants at various MTBD-microtubule distances (see legend). In each panel, the number of contacts was normalized by the total number of contacts each particular distance over the 3 independent runs of 2000 total frames each (see Methods). Note that no results are shown here for distances larger than 35 Å because there are few contacts, however these numbers are provided in supplementary material. The right panels show the MTBD at distance = 15 Å with electrostatic potential mapped onto its surface, and the α-/β- tubulin dimer with A, B, C, D chains E-hooks highlighted (red). Note, a frame was chosen in which the E-hooks are shown not making contact with the MTBD for clarity. The residues of MTBD making most of the contacts with E-hooks are labeled.