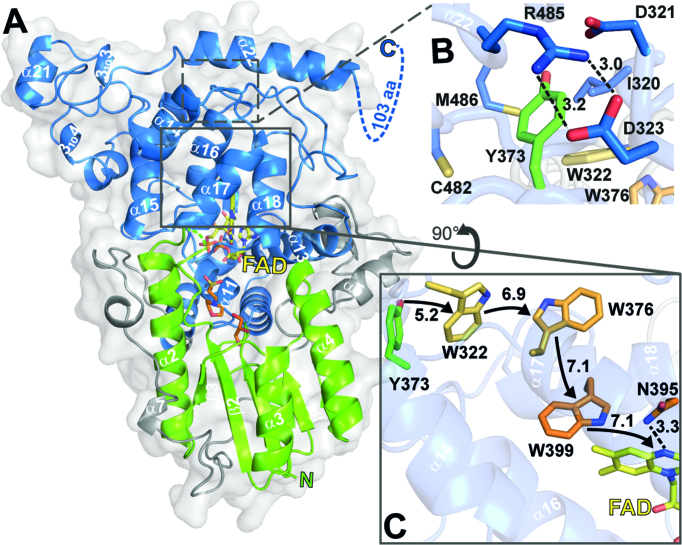
Figure 1.
Cartoon representation of CraCRY structure with highlighted regions of interest. (A) Overall structure of CraCRYΔCTE (6FN3) with FAD (yellow) bound to the C-terminal domain (blue). The C- and N-terminal domain (green) are connected through a flexible loop region (grey). The antenna binding site is occupied by glycerol (GOL, orange) and 2-(N-morpholino) ethanesulfonic acid (MES, orange). The C-terminal extension (494–595) is displayed as dotted lines. (B) Detail environment of the fourth aromatic residue, Y373. (C) The electron transfer pathway of CraCRY is marked with arrows, centroid-centroid distances are shown in Å. The Trp triad is shown in orange and the fourth aromatic residue, a terminal tyrosine (Y373), is shown in green. Also the potential proton donorof the FAD, N395, is presented as sticks.