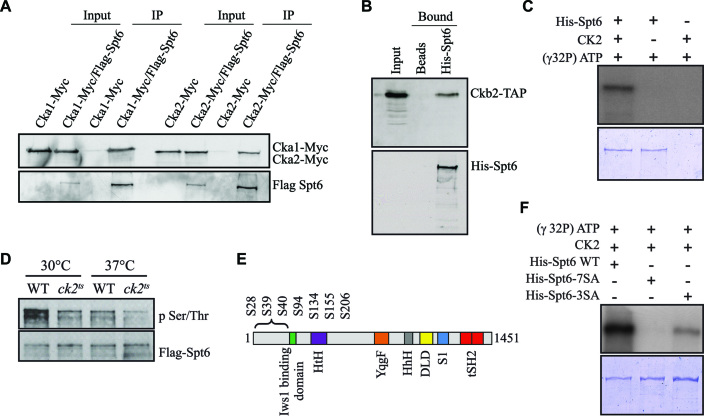
Figure 3.
CK2 interacts with Spt6 and phosphorylates it in vitro and in vivo. (A) Spt6 interacts with different subunits of CK2 in vivo. Flag-Spt6 was immunopurified from strains expressing Cka1-Myc or Cka2-Myc. The purified samples were analyzed by western blot with antibodies against the Myc and Flag epitopes. A purification control was obtained using strains expressing untagged Spt6. (B) Spt6 interacts directly with CK2 in vitro. A His pulldown assay was performed using equal amounts of Ckb2-TAP purified from yeast and recombinant 6xHis-Spt6. Ni-NTA agar beads alone served as a control. (C) CK2 phosphorylates Spt6. In vitro phosphorylation assay of recombinant 6xHis-Spt6 with CK2 kinase purified from yeast. (D) Spt6 is phosphorylated in vivo in a CK2-dependent manner. Flag-Spt6 was immunopurified from WT and ck2ts strains grown at 30°C or at 37°C for 2 h. The purified samples were analyzed by western blot with antibodies against phosphoserine/phosphothreonine or Flag epitope. (E) Schematic representation of various domains of Spt6: potential CK2 target sites (S28, S39, S40, S94, S134, S155, S206) are all found in the N-terminal region. (F) The N-terminal phosphosites of Spt6 (S28, S39, S40, S94, S134, S155, S206) are required for the phosphorylation by yeast CK2. In vitro phosphorylation assay of recombinant 6xHis-Spt6 WT, and non-phosphorylatable mutants (6xHis-Spt6-3SA and -7SA) using CK2 kinase purified from yeast.